Different types of COVID-19 vaccines: How they work
Understand how different vaccine technologies work with the immune system to provide protection.
By Mayo Clinic Staff
COVID-19 vaccines lower your risk of getting sick, seriously ill or dying from the disease. But how do the different types of COVID-19 vaccines work?
Each COVID-19 vaccine causes the immune system to create proteins called antibodies. These proteins fight infection with the COVID-19 virus. COVID-19 vaccines use a harmless version of a spikelike structure called an S protein on the surface of the COVID-19 virus.
There are a few main types of COVID-19 vaccines.
Messenger RNA (mRNA) vaccine
mRNA vaccine
mRNA vaccine
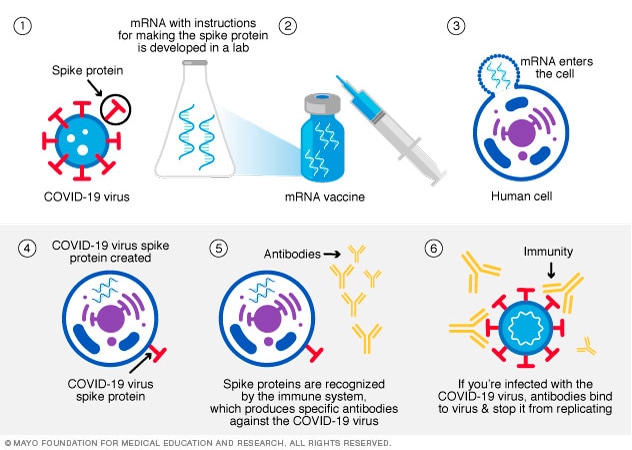
An mRNA vaccine gives cells instructions for how to make the S protein found on the surface of the COVID-19 virus. After vaccination, the body's muscle cells begin making the protein pieces and showing them on cell surfaces. This causes the body to create antibodies. Then if you catch the COVID-19 virus, these antibodies are used to help clear out the virus.
Once the protein pieces are made, your cells break down the instructions and get rid of them. The mRNA in the vaccine doesn't enter the nucleus of the cell, where DNA is kept. Both the Pfizer-BioNTech and the Moderna COVID-19 vaccines use mRNA.
Vector vaccine
Viral vector vaccine
Viral vector vaccine
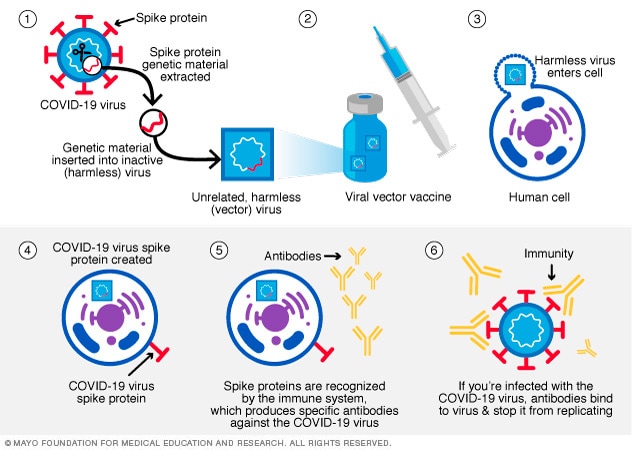
In this type of vaccine, material from the virus that causes COVID-19 is placed in a modified version of a different virus. This different virus is called a viral vector. The viral vector gives cells instructions to make copies of the COVID-19 virus S protein.
Once the cells display the S proteins on their surfaces, the immune system responds by creating antibodies and defensive white blood cells. If infection with the virus that causes COVID-19 happens later, the antibodies help clear out the virus.
Viral vector vaccines can't cause infection with the COVID-19 virus or the viral vector virus. The Johnson & Johnson COVID-19 vaccine is a vector vaccine that's no longer used in the United States.
Protein subunit vaccine
Protein subunit vaccine
Protein subunit vaccine
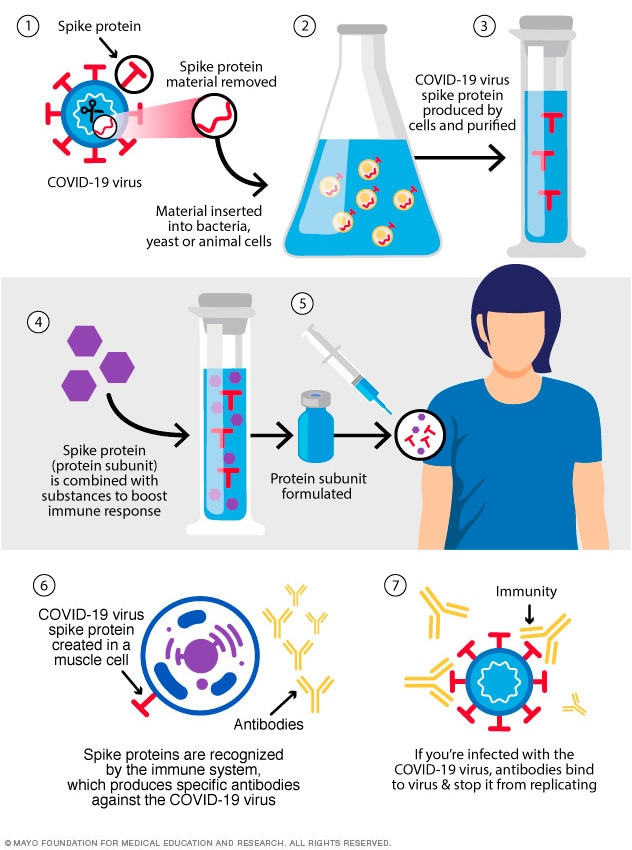
Subunit vaccines include only the parts of a virus that best stimulate the immune system. This type of COVID-19 vaccine has harmless S proteins in it. Once the immune system recognizes the S proteins, it creates antibodies and defensive white blood cells. If infection with the COVID-19 virus happens later, the antibodies help clear out the virus.
The Novavax COVID-19 vaccine is a protein subunit vaccine.
COVID-19 vaccines
The COVID-19 vaccines available in the United States are:
- Pfizer-BioNTech COVID-19 vaccine 2024-2025 formula, available for people age 6 months and older.
- Moderna COVID-19 vaccine 2024-2025 formula, available for people age 6 months and older.
- Novavax COVID-19 vaccine, adjuvanted 2024-2025 formula, available for people age 12 years and older.
Getting vaccinations as they are updated and on schedule gives you the best protection against COVID-19.
Jan. 30, 2025
- COVID-19 vaccine basics. Centers for Disease Control and Prevention. https://www.cdc.gov/covid/vaccines/how-they-work.html. Accessed Oct. 11, 2024.
- The different types of COVID-19 vaccines. World Health Organization. https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained. Accessed Feb. 3, 2021.
- Edwards KM, et al. COVID-19: Vaccines. https://www.uptodate.com/contents/search. Accessed Oct. 11, 2024.
- Amanat F, et al. SARS-CoV-2 vaccines: Status report. Immunity. 2020; doi:10.1016/j.immuni.2020.03.007.
- Staying up to date with COVID-19 vaccines. Centers for Disease Control and Prevention. https://www.cdc.gov/covid/vaccines/stay-up-to-date.html. Accessed Oct. 11, 2024.
- Vaccine types. U.S. Department of Health and Human Services. https://www.hhs.gov/immunization/basics/types/index.html. Accessed Feb. 2, 2021.
- Vaccine types. National Institute of Allergy and Infectious Diseases. https://www.niaid.nih.gov/research/vaccine-types. Accessed Feb. 2, 2021.
- Real-time learning network vaccines FAQ. Infectious Diseases Society of America. https://www.idsociety.org. Accessed Oct. 11, 2024.
- Vaxzevria (previously COVID-19 Vaccine AstraZeneca). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria. Accessed Oct. 11, 2024.