Different types of COVID-19 vaccines: How they work
Understand how different vaccine technologies work with the immune system to provide protection.
By Mayo Clinic Staff
COVID-19 vaccines lower the risk of getting sick, seriously ill or dying from the disease. But how do the different types of COVID-19 vaccines work?
Each COVID-19 vaccine causes the immune system to create proteins called antibodies. These proteins fight infection with the COVID-19 virus. COVID-19 vaccines use a harmless version of a spikelike structure called an S protein on the surface of the COVID-19 virus.
There are a few main types of COVID-19 vaccines.
Messenger RNA (mRNA) vaccine
mRNA vaccine
mRNA vaccine
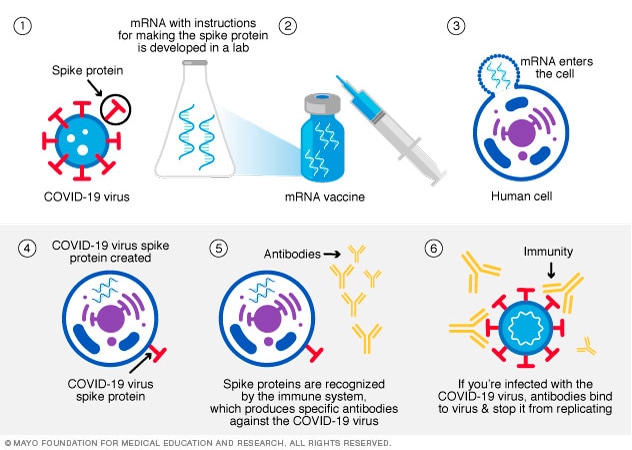
An mRNA vaccine gives cells instructions for how to make the S protein found on the surface of the COVID-19 virus.
The vaccine shot sends the mRNA into the muscle. Your muscle cells may take in the mRNA and make the S protein. Or the mRNA may be picked up by an immune cell. Either the mRNA or the S protein alerts the body to the risk of the virus. Then the body begins to create a response to prevent you from getting sick from the infection.
Vector vaccine
Viral vector vaccine
Viral vector vaccine
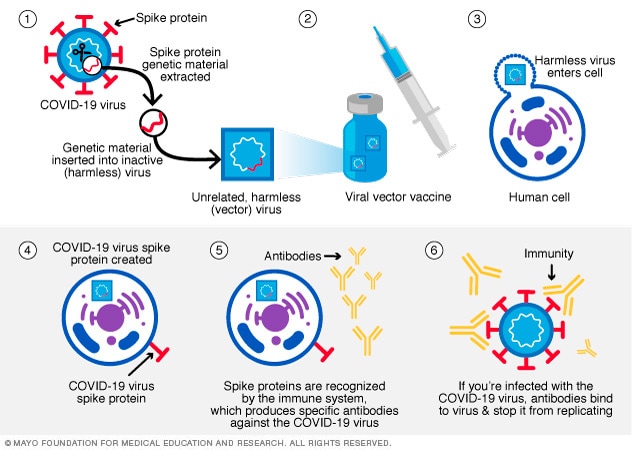
In this type of vaccine, material from the virus that causes COVID-19 is placed in a modified version of a different virus. This different virus is called a viral vector. The viral vector gives cells instructions to make copies of the COVID-19 virus S protein.
Once the cells display the S proteins on their surfaces, the immune system responds by creating antibodies and defensive white blood cells. If infection with the virus that causes COVID-19 happens later, the antibodies help clear out the virus.
Viral vector vaccines can't cause infection with the COVID-19 virus or the viral vector virus. The Johnson & Johnson COVID-19 vaccine is a vector vaccine that's no longer used in the United States.
Protein subunit vaccine
Protein subunit vaccine
Protein subunit vaccine
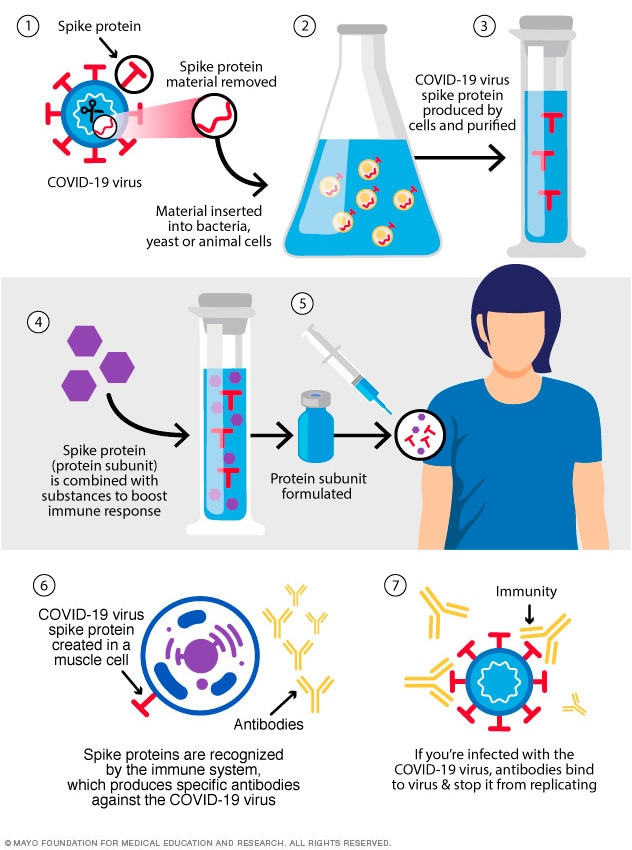
Subunit vaccines include only the parts of a virus that best stimulate the immune system. This type of COVID-19 vaccine has harmless S proteins in it. Once the immune system recognizes the S proteins, it creates antibodies and defensive white blood cells. If infection with the COVID-19 virus happens later, the antibodies help clear out the virus.
The Novavax COVID-19 vaccine is a protein subunit vaccine.
COVID-19 vaccines
The 2025-2026 COVID-19 vaccines available in the United States include Moderna, Pfizer-BioNTech and Novavax. The vaccines are all approved for people age 65 and older. Each vaccine is also approved for younger people with a health condition that raises the risk of serious COVID-19 illness.
Moderna COVID-19 vaccine 2025-2026 formulas.
- Spikevax. This vaccine is approved for people age 6 months to 64 years with a risk of serious COVID-19 illness and all people age 65 and older.
- Mnexspike. This vaccine is approved for people age 12 to 64 years with a risk of serious COVID-19 illness and all people age 65 and older.
Pfizer BioNTech COVID-19 vaccine 2025-2026 formula.
- Comirnaty. This vaccine is approved for people age 5 to 64 years with a risk of serious COVID-19 illness and all people age 65 and older.
Novavax COVID-19 vaccine 2025-2026 formula.
- Nuvaxovid. This vaccine is approved for people age 12 to 64 years with a risk of serious COVID-19 illness and all people age 65 and older.
The Pfizer-BioNTech and Moderna COVID-19 vaccines for 2025-2026 focus on building protection against the LP.8.1 virus strain. The Novavax COVID-19 vaccine helps build protection against the JN.1 strain.
Who should get a COVID-19 vaccine?
Getting a COVID-19 vaccine protects against serious illness, the need for hospital care due to COVID-19 and death from COVID-19. Staying up to date with the latest vaccine is most important for people at higher risk. That includes adults over age 65, those with weakened immune systems, people who are pregnant, and people with chronic conditions, such as heart disease, lung disease or obesity.
History of infectious disease outbreaks and vaccines timeline.
Learn about the history of major disease outbreaks, epidemics and pandemics, as well as the impact vaccines and research had on many infectious diseases.
Find out more at History of infectious disease outbreaks and vaccines timeline.
Dec. 24, 2025
- COVID-19 vaccine basics. Centers for Disease Control and Prevention. https://www.cdc.gov/covid/vaccines/how-they-work.html. Accessed Oct. 11, 2024.
- The different types of COVID-19 vaccines. World Health Organization. https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained. Accessed Feb. 3, 2021.
- Edwards KM, et al. COVID-19: Vaccines. https://www.uptodate.com/contents/search. Accessed Oct. 11, 2024.
- Amanat F, et al. SARS-CoV-2 vaccines: Status report. Immunity. 2020; doi:10.1016/j.immuni.2020.03.007.
- Staying up to date with COVID-19 vaccines. Centers for Disease Control and Prevention. https://www.cdc.gov/covid/vaccines/stay-up-to-date.html. Accessed Oct. 11, 2024.
- Vaccine types. U.S. Department of Health and Human Services. https://www.hhs.gov/immunization/basics/types/index.html. Accessed Feb. 2, 2021.
- Vaccine types. National Institute of Allergy and Infectious Diseases. https://www.niaid.nih.gov/research/vaccine-types. Accessed Feb. 2, 2021.
- Real-time learning network vaccines FAQ. Infectious Diseases Society of America. https://www.idsociety.org. Accessed Oct. 11, 2024.
- Vaxzevria (previously COVID-19 Vaccine AstraZeneca). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria. Accessed Oct. 11, 2024.
- Pardi N, et al. mRNA vaccines for infectious diseases — Advances, challenges and opportunities. Nature Reviews Drug Discovery. 2024; doi:10.1038/s41573-024-01042-y.
- Spikevax (approval letter). Biologic License Application 125752. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/spikevax. Accessed Sept. 25, 2025.
- Mnexspike (approval letter). Biologic License Application 125835 U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/mnexspike. Accessed Sept. 25, 2025.
- Comirnaty (approval letter). Biologic License Application 125742. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/comirnaty. Accessed Sept. 25, 2025.
- Nuvaxovid (approval letter). Biologic License Application 125817. U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/vaccines/nuvaxovid. Accessed Sept. 25, 2025.
- Medical review (expert opinion). Mayo Clinic. Sept. 25, 2025.