April 19, 2019
Results of the National Emphysema Treatment Trial (NETT), published in New England Journal of Medicine in 2003, convincingly demonstrated improvement in lung function and symptomatic relief of patients with severe emphysema with lung volume reduction surgery (LVRS).
This benefit was evident only when LVRS was performed on selected patients with upper lobe predominant emphysema and a low baseline exercise capacity. While effective, the LVRS procedure helps only a select patient population with severe emphysema and is associated with a high cost, the potential for postoperative surgical complications and related lengthy hospital stays, and other surgical risks.
Recognition of the benefits and risks associated with LVRS prompted further evaluation and investigation of less invasive techniques to achieve lung volume reduction. Bronchoscopic lung volume reduction has emerged as a potential alternative to LVRS, and a less invasive method by which to achieve lung volume reduction in patients with emphysema and hyperinflation.
Studies assessing bronchoscopic therapies aimed at reducing lung volumes have evaluated the use of various one-way valves that cause distal atelectasis, lung volume reduction coils that mechanically reduce lung volume, and thermal ablation. Initial studies had mixed results, though both endobronchial coils and one-way valves are commercially available for the treatment of emphysema in many countries worldwide outside the United States.
Bronchoscopy treatment for emphysema
Zephyr Endobronchial Valve System
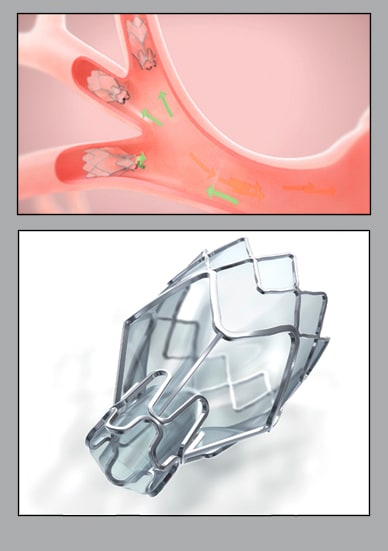
Zephyr Endobronchial Valve System
Endobronchial Valve System positioned in lung airway (top). Zephyr Endobronchial Valve System (bottom). Zephyr Valve, courtesy of Pulmonx Corp.
In June 2018, the FDA approved Zephyr endobronchial valves, manufactured by Pulmonx, as the first bronchoscopic treatment for emphysema in the United States for patients with hyperinflation and minimal collateral ventilation. The role of these endobronchial valves in the management of emphysema was evaluated in the LIBERATE study published in American Journal of Respiratory and Critical Care Medicine in 2018.
LIBERATE included 160 patients and randomized 128 to treatment with endobronchial valves and compared them with 62 patients treated by standard of care (optimized bronchodilator therapy and pulmonary rehabilitation).
LIBERATE included patients who were former smokers between the ages of 40 and 75 with a post-bronchodilator forced expiratory volume in one second (FEV1) between 15 and 45 percent, and with hyperinflation as evidenced by a residual volume ≥ 175 percent predicted. They also had a diffusing capacity of the lungs for carbon monoxide ≥ 20 percent and a six-minute walk distance of 100 to 500 m. A CT had to reveal heterogeneous emphysema.
Eligible patients then underwent bronchoscopy using the Chartis system to identify collateral ventilation. At 12 months, 47.7 percent of valve recipients and 16.8 percent of the control arm had an increase in FEV1 ≥ 15 percent (p < 0.001).
Further, valve recipients had significant improvements in their six-minute walk distances (+ 39.31 m; p = 0.002) and improvements in dyspnea as measured by the St. George's Respiratory Questionnaire (-7.05 points; p = 0.004). The major side effect with endobronchial valve placement was a pneumothorax, occurring in 27 percent of recipients, with 85 percent occurring within the first five days.
Similar to LVRS, this procedure will likely benefit only a subset of patients with emphysema. For this trial, 909 patients gave consent. Only 160 met the full inclusion trial. Of those patients who did not meet the inclusion criteria:
- 40 percent were excluded due to imaging characteristics predictive of suboptimal responses
- 22 percent were excluded for lung volumes
- 9 percent were excluded for collateral ventilation found on bronchoscopy after passing the noninvasive criteria
Despite inclusion criteria limitations, there may still be a sizable population of patients with emphysema who may benefit from this therapy. Mayo Clinic now offers this procedure for carefully selected patients.
For more information
National Emphysema Treatment Trial (NETT). National Heart, Lung, and Blood Institute.
Fishman A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. New England Journal of Medicine. 2003;348:2059.
Criner GJ, et al. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). American Journal of Respiratory and Critical Care Medicine. 2018;198:1151.