June 19, 2021
The Food and Drug Administration (FDA) has approved the use of three medications for the treatment of neuromyelitis optica spectrum disorder (NMOSD). Mayo Clinic led the biomarker discovery and subsequent epidemiologic, immunopathological, clinical and radiologic phenotyping of this debilitating inflammatory central nervous system disorder.
"NMOSD is considered an orphan disease. The fact that we have three FDA-approved drugs within a year of one another is pretty incredible," says Sean J. Pittock, M.D., an autoimmune neurologist who directs the Neuroimmunology Research Laboratory and the Center for Multiple Sclerosis and Autoimmune Neurology at Mayo Clinic in Rochester, Minnesota.
Lesions in the AQP4-enriched postrema
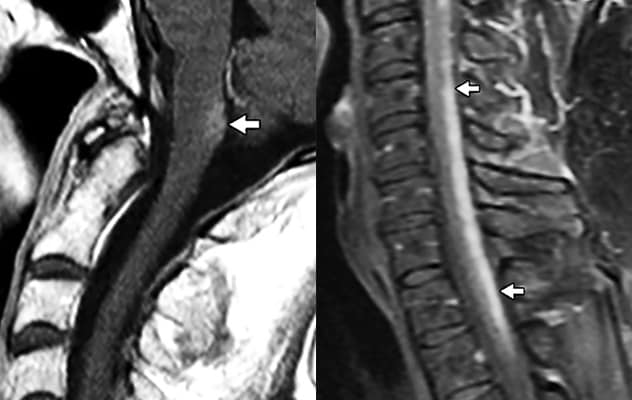
Lesions in the AQP4-enriched postrema
On the left, MRI shows lesions in the AQP4-enriched postrema, characteristic of some patients with NMOSD. On the right, MRI of a patient with sarcoidosis illustrates the differing imaging characteristics of the two conditions.
NMOSD manifests primarily as relapsing episodes of severe optic neuritis and longitudinally extensive transverse myelitis. Historically misdiagnosed as multiple sclerosis, NMOSD is characterized by more-severe attacks and less complete recovery. The median age of onset is 35 to 37 years.
"There's always a possibility that an attack could leave the person with a deficit such as blindness in an eye or difficulty walking," says Dean M. Wingerchuk, M.D., an autoimmune neurologist at Mayo Clinic in Phoenix/Scottsdale, Arizona. "The attacks are also very unpredictable. Patients always live with the specter of drastic change that could happen to them quickly."
Unlike multiple sclerosis, in which morbidity generally accrues as part of the disease's disability phase, NMOSD has cumulative effects. "Each NMOSD attack leads to additional disability for most patients," says Alfonso (Sebastian) S. Lopez Chiriboga, M.D., an autoimmune neurologist at Mayo Clinic in Jacksonville, Florida. "In rare instances, when severe inflammation causes upper cervical cord lesions, patients can succumb to respiratory failure."
NMOSD has traditionally been treated with immunosuppressants. However, controlled studies have been lacking, and up to half of patients continue to experience attacks while receiving these therapies. The newly approved treatments are three monoclonal antibodies:
- Eculizumab, a complement inhibitor
- Inebilizumab, an anti-CD19 agent
- Satralizumab, an anti-interleukin-6 receptor
"They're all excellent therapies. They're also quite different from one another in some respects," Dr. Wingerchuk says. "Our neurologists have seen hundreds of people with NMOSD. That experience is very helpful in counseling patients about treatment."
Mayo Clinic has pioneered the research and clinical management of NMOSD for more than 20 years. The discovery of an antibody biomarker by a team led by Vanda A. Lennon, M.D., Ph.D., an immunologist at Mayo Clinic in Minnesota, revolutionized the diagnosis and treatment of NMOSD.
Subsequent studies of the antibody — known as neuromyelitis optica antibody (NMO-IgG) — and its target, the aquaporin-4 (AQP4) water channel — faciliated improved understanding of the immunopathological mechanisms underlying the disorder and allowed the development of novel therapies. Although considered rare, AQP4-IgG-seropositive NMOSD affects about half a million people worldwide, disproportionately nonwhite and women.
Drs. Pittock and Wingerchuk led a Mayo Clinic trial of eculizumab, published in The Lancet in 2013, that demonstrated nearly complete cessation of disease activity in patients severely affected by NMOSD, paving the way for eculizumab's phase 3 clinical trial. In addition, Drs. Pittock and Wingerchuk served with Brian G. Weinshenker, M.D., a neurologist at Mayo Clinic's campus in Minnesota, on the steering committee of the clinical trial investigating inebilizumab for the treatment of NMOSD.
"The fact that we now have three medications that target the specific disease pathways in patients with the aquaporin-4 antibody is a game changer," Dr. Lopez Chiriboga says.
Therapeutic decision-making
None of the newly approved treatments caused major side effects among trial participants. Beyond that, direct comparison of the clinical trial results is difficult due to the trials' differing designs and definitions.
Mayo Clinic autoimmune neurologists advise physicians and patients to consider each therapy's efficacy, convenience and cost:
- In the PREVENT eculizumab trial, published in The New England Journal of Medicine in 2019, 98% of patients receiving the therapy were relapse-free 144 weeks after starting treatment, compared with 45% in the placebo group. Eculizumab must be infused at a medical center every two weeks and costs about $710,000 a year.
- In the N-MOmentum inebilizumab trial, published in The Lancet in 2019, 88% of patients receiving the therapy were relapse-free 28 weeks after starting treatment, compared with 61% in the placebo group. Inebilizumab must be infused at a medical center every six months. It costs $393,000 the first year and $262,000 a year after that.
- In the SAkuraStar/SAkuraSky satralizumab trials, published in The New England Journal of Medicine in 2019 and The Lancet in 2020, approximately 78% of patients receiving the therapy were relapse-free 96 weeks after starting treatment, compared with 59% in the placebo group. Satralizumab is injected under the skin at home once a month. It costs $219,000 the first year and $190,000 a year after that.
Rituximab, a monoclonal antibody often used to treat NMOSD, costs about $18,000 a year. The cost continues to decline, as the medication is now off patent. Regulatory approval hasn't been sought for rituximab as an NMOSD treatment.
Dr. Pittock notes that a recent randomized controlled trial at a single institution in Japan found that rituximab was more effective than a placebo. Although the study's small size precludes meaningful quantification of risk, "it's difficult to ignore rituximab," Dr. Pittock says.
Mayo Clinic is currently a site for a pharmaceutical company-funded study investigating ravulizumab, a monoclonal antibody inhibitor of complement activation that is similar to eculizumab but with a longer half-life. "Potentially, this therapy would need to be given only every eight weeks," Dr. Pittock says.
Mayo Clinic's leadership in complex diseases such as NMOSD stems from a commitment to innovation. In addition to laboratory studies of the disease's underlying mechanisms, diagnostic assays are developed through Mayo Clinic Laboratories. The positive predictive value for Mayo's NMO/AQP4-IgG assays is 100%.
The Mayo Clinic Neuroimmunology Research Laboratory discovers approximately two new antibody biomarkers of autoimmune or paraneoplastic neurological disorders a year. "We are making giant leaps in moving the field of autoimmune neurology forward. This allows us to provide molecular target-based diagnoses, which opens up the field for personalized targeted immunotherapies," Dr. Pittock says.
"At Mayo Clinic, our work goes from bench to bedside — from biomarker discovery to treatments that can stop attacks," he adds. "By stopping NMOSD attacks, we can potentially stop the accrual of disability."
For more information
Center for Multiple Sclerosis and Autoimmune Neurology. Mayo Clinic.
Pittock SJ, et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: An open-label pilot study. The Lancet. 2013;12:554.
Pittock SJ, et al. Eculizumab in Aquaporin-4-positive neuromyelitis optica spectrum disorder. The New England Journal of Medicine. 2019;381:614.
Cree BAC, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): A double-blind, randomised placebo-controlled phase 2/3 trial. The Lancet. 2019;394:1352.
Yamamura T, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. The New England Journal of Medicine. 2019;381:2114.
Traboulsee A, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: A randomised, double-blind, multicentre, placebo-controlled phase 3 trial. The Lancet. 2020;19:402.