May 25, 2018
A 34-year-old woman was referred for evaluation of ovarian enlargement and a pituitary mass. She had normal childhood development and regular menses until age 33, when her cycles became more frequent. She and her husband had been trying to conceive for approximately five years, but were unsuccessful.
Three months prior to her presentation at Mayo Clinic, the patient developed pelvic pain and evaluation revealed marked enlargement of her right ovary (10 cm) with 12 cysts and a serum estradiol of 1,790 pg/mL (reference range, 15 to 350 pg/mL). She underwent right oophorectomy, but developed recurrent pelvic pain six weeks later, prompting an ultrasound that showed a 4.6-cm cyst on the left ovary.
She was treated with leuprolide 3.75 mg and 12 hours later her luteinizing hormone (LH) was 26.7 IU/L (reference range, 1.2 to 12.9 IU/L) and follicle-stimulating hormone (FSH) was 43.9 IU/L (reference range, 1.8 to 8.8 IU/L).
Head MRI scan
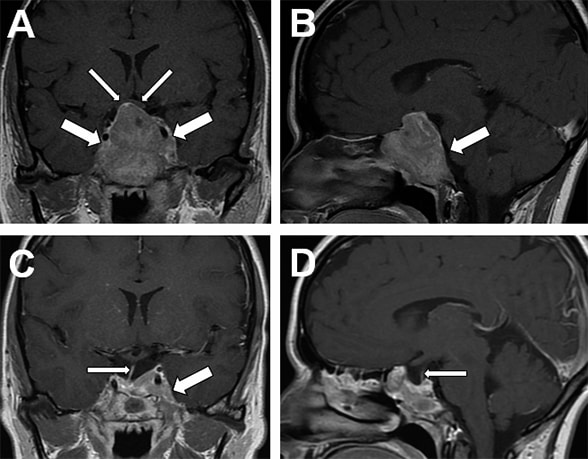
Head MRI scan
A. Coronal image showing pituitary macroadenoma (large arrows) extending into the suprasellar cistern, elevating the optic chiasm (small arrows), and involving sphenoid sinus and both cavernous sinuses. B. Sagittal image showing extension into the clivus (arrow). C. Three-month post-surgery head MRI coronal image shows debulking of the pituitary macroadenoma, visible pituitary stalk (small arrow), and residual tumor in the left cavernous sinus (large arrow). D. Three-month postoperative sagittal MRI shows a secondary empty sella and a normal pituitary stalk (small arrow).
Two days after receiving leuprolide, the patient developed a severe headache associated with photophobia and vomiting. Head MRI showed a sellar mass (4.7 by 5.1 by 3.7 cm) extending into the suprasellar cistern, cavernous sinuses, clivus and sphenoid sinus.
On examination at Mayo Clinic, the patient appeared well, but had a palpable mass in her lower abdomen. Laboratory evaluation showed the following:
- Serum estradiol, 3,740 pg/mL
- LH, 36 IU/L; FSH, 38.7 IU/L
- Prolactin, 115 ng/mL (reference range, 4.8 to 23.3 ng/mL)
- Normal blood concentrations of cortisol, insulin-like growth factor-I, testosterone, thyroid-stimulating hormone (TSH), free T4 and beta-human chorionic gonadotropin (βHCG)
Preoperative and postoperative pelvic ultrasounds
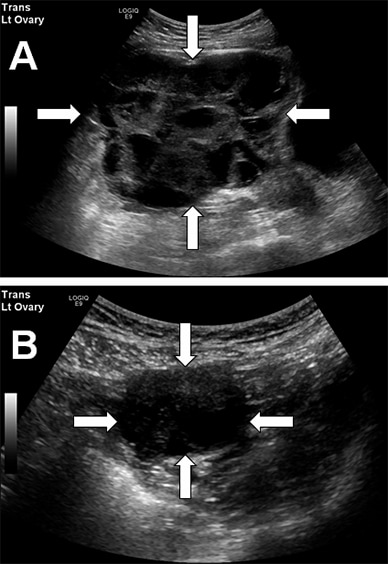
Preoperative and postoperative pelvic ultrasounds
A. Preoperative pelvic ultrasound shows a large (12.2 by 14.4 by 14.5 cm, volume 1,321 mL) left ovary with innumerable hemorrhagic cysts. B. Pelvic ultrasound three months after pituitary surgery shows marked reduction in left ovarian size (3.5 by 3.9 by 4 cm, volume 29 mL) and number of follicles.
Pelvic ultrasound revealed a markedly enlarged left ovary with innumerable hemorrhagic cysts.
The patient underwent transsphenoidal surgical debulking of the pituitary tumor without complications. Immunohistochemistry findings were consistent with a gonadotroph adenoma, staining weakly for LH alpha-subunit, and FSH beta-subunit, and strongly for chromogranin. The Ki-67 labeling index was less than 2 percent. Laboratory studies the day after surgery showed the following:
- LH, 6.8 IU/L
- FSH, 10.5 IU/L
- Estradiol, 2,190 pg/mL
During the first postoperative month, LH and FSH remained normal, but estradiol fluctuated between 2,000 and 4,000 pg/mL as the patient's left ovary decreased in size. At 1.5 months post-surgery, estradiol normalized to 19 pg/mL with LH equal to 1.9 IU/L and FSH equal to 2.8 IU/L. Three months after surgery, the patient had return of menses and pelvic ultrasound showed marked reduction in ovarian size. Head MRI showed debulking of the pituitary macroadenoma with residual tumor in the left cavernous sinus.
Because the residual tumor was in close proximity to the left optic nerve, the patient was not a candidate for stereotactic radiotherapy. She received fractionated radiation to prevent future tumor growth; six months later, she became pregnant.
This case illustrates the clinical course of a functioning gonadotroph adenoma causing ovarian hyperstimulation. As described in a review published in the Journal of Clinical Endocrinology and Metabolism in 2014, functional gonadotroph adenomas are rare pituitary tumors that secrete biologically active gonadotropins and cause distinct clinical manifestations. Premenopausal women may experience menstrual irregularities, infertility, galactorrhea, headaches, visual disturbances from mass effect and ovarian hyperstimulation.
Laboratory findings include hyperestrogenism (which may range from mild to marked), elevated or inappropriately normal FSH, variable LH levels, and hyperprolactinemia due to pituitary stalk compression or elevated estradiol levels. When ovarian hyperstimulation occurs, pelvic ultrasound demonstrates bilateral ovarian enlargement with multiseptated cysts of variable sizes.
Most of the reported functioning gonadotroph adenomas have been macroadenomas, often invading the cavernous sinuses and distorting the optic chiasm via suprasellar extension. These rare pituitary adenomas have also been reported in men, sometimes causing testicular enlargement, and rarely in children, causing precocious puberty.
Like other nonprolactinoma pituitary adenomas, surgery is the mainstay of therapy for functioning gonadotroph adenomas. If successful, surgery may lead to normalization of gonadotropin secretion and resolution of symptoms, but surgery alone may not be curative for the larger, more-invasive tumors. Residual tumor has been treated with radiotherapy in some cases, but long-term follow-up data evaluating efficacy are lacking.
Several medical therapies, including dopamine agonists, somatostatin analogues, and Gn-RH agonists and antagonists have been used, but none of these has been associated with tumor shrinkage. While dopamine agonists and somatostatin analogues have decreased ovarian volumes and gonadotropin secretion in a limited number of cases, Gn-RH antagonists have been associated with mixed results, and Gn-RH agonists have led to increased gonadotropin secretion and exacerbation of ovarian hyperstimulation syndrome.
While there is little long-term data to guide ongoing management of our patient, we plan on monitoring for tumor regrowth on an annual basis.
For more information
Ntali G, et al. Functioning gonadotroph adenomas. Journal of Clinical Endocrinology and Metabolism. 2014;99:4423.