Nov. 05, 2019
Rhabdomyosarcoma (RMS) represents 3% of all childhood malignancies with approximately 350 new cases a year in the United States. Research published in the Journal of Surgical Research in 2011 supports that genitourinary RMS comprises approximately 20% of all cases. In those cases of RMS with bladder or prostate origin, which are deemed unfavorable sites, a worse prognosis is portended.
Although alterations in the composition and duration of chemotherapy for RMS have evolved, the agents used remain largely unchanged since the 1970s. These drugs have significant side effects, and there is ongoing concern for long-term deleterious effects in survivors. As noted in research published in the Journal of Clinical Oncology in 2001, of utmost concern is that current treatment plans still have abysmal three-year failure-free survival rates for stage III disease inclusive of the bladder and prostate, with worse outcomes in infants. Infants presenting with metastatic disease having the lowest overall survival rate (20% to 30%).
Pediatric urologists at Mayo Clinic's campus in Rochester, Minnesota, are on a mission to improve outcomes, particularly for those patients who do not respond to standard treatment regimens or have intra-abdominal pelvic relapse.
"Survival is directly correlated with the ability to completely resect all disease. Tackling these rare tumors in a multidisciplinary fashion with an experienced sarcoma surgical team is critical," says Candace F. Granberg, M.D. "Even with an experienced team, microscopic spread is inevitable and requires treatment of the entire abdominal cavity. Many of these children have often reached their maximum dose of radiotherapy, or parents hope to avoid whole-abdominal radiotherapy due to the known and often severe side effects."
Peritoneal cavity treated without radiation
Mayo Clinic is spearheading a new phase I clinical trial of adjuvant surgical treatment with intraperitoneal chemotherapy that specifically addresses patients with relapsed intra-abdominal and pelvic rhabdomyosarcoma. This prospective study explores the effects of hyperthermic intraperitoneal chemotherapy (HIPEC) using doxorubicin and cisplatin after employment of complete cytoreductive surgery (CCS). Mayo Clinic is the only center in the United States to offer this trial.
Hyperthermic intraperitoneal chemotherapy (HIPEC) setup
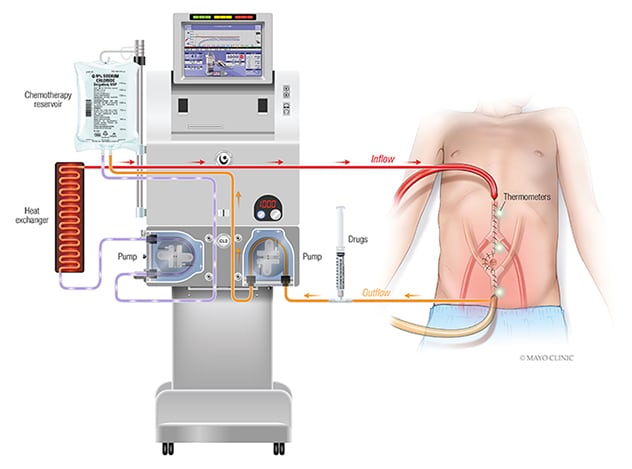
Hyperthermic intraperitoneal chemotherapy (HIPEC) setup
Hyperthermic intraperitoneal chemotherapy (HIPEC) involves administration of a highly concentrated, heated chemotherapy solution directly into the abdomen intraoperatively after complete removal of all visible tumor.
HIPEC involves administration of a highly concentrated, heated chemotherapy solution directly into the abdomen intraoperatively after complete removal of all visible tumor. Unlike systemic chemotherapy, which circulates throughout the body, HIPEC delivers chemotherapy directly to any remaining cancer cells in the abdomen. This delivery method also allows for higher treatment doses. "It is hypothesized that by heating the chemotherapy solution, absorption of drugs by tumors with resultant tumor cell death may be improved," says Patricio C. Gargollo, M.D.
Mayo Clinic's multidisciplinary, dedicated team of high-volume pediatric sarcoma surgeons, chemoperfusionists and pediatric anesthesiologists collaborate during the CCS plus HIPEC operation itself. Postoperatively, an integrated team of specialists in pediatric critical care, pediatric pain, pediatric gastroenterology and nutrition, in addition to nursing staff, provides the meticulous care and monitoring required. Mayo Clinic's approach to CCS plus HIPEC is detailed in an article published on Mayo Clinic's Medical Professionals website and featured in Cancer Physician Update e-Edition in February 2018.
Precision medicine for pediatric RMS
At Mayo Clinic, innovation does not stop at surgical resection. Researchers work diligently to determine why some tumors respond to standard combination chemotherapy and radiation while others grow during treatment, recur or metastasize ― and what key role immunotherapy may play.
"Since the introduction of immunotherapy to the armamentarium of oncologists, unprecedented clinical responses have been observed in adult cancers. However, immunotherapy is still in its infancy in pediatric RMS, thus significant efforts are required to characterize the tumor immune microenvironment and assess the clinical utility of immunotherapy," says Dr. Granberg.
"By studying tissue removed during surgery, we are able to identify tumor-specific antigens expressed at the cell surface of RMS tumor cells. Aberrant expression of cell-surface proteins may represent an important source of anti-cancer targets since their extracellular accessibility makes therapeutic intervention more effective with immunotherapy," says Dr. Gargollo.
Research process
The research team used cell-surface isolation and targeted proteomic profiling by mass spectrometry to analyze the surfaceome of fusion-positive, fusion-negative patient-derived RMS cells and normal skeletal muscle. They performed high-throughput protein analysis through several protein databases to identify evolutionary relationships, enriched molecular functions and biological processes. Flow cytometry and immunohistochemistry of tumor specimens were performed to confirm expression of targetable cell-surface antigens.
"This work provides novel insights in regards to RMS development and progression. Top cell-surface candidates represent promising targets for drug therapy, and development of novel treatment approaches will expand the therapeutic landscape of children diagnosed with RMS," says Dr. Granberg.
Working with Haidong Dong, M.D., Ph.D., a urologist at Mayo Clinic in Rochester, Minnesota, the research team, led by Fabrice Lucien-Matteoni, Ph.D., was able to determine expression profiles of B7 immune checkpoint molecules by flow cytometry of patient-derived RMS cells. "We found that B7-H3, an immune checkpoint molecule, is constitutively expressed in pediatric RMS and contributes to immunosuppression and disease progression," says Dr. Dong. "Importantly, it was noted that blockade of B7-H3 restores anti-tumor immune response in vitro and in vivo."
"This work highlights the therapeutic potential of targeting B7-H3 and supports the implementation of immunotherapy for the treatment of pediatric RMS. With innovative surgical approaches and collaborative research efforts, individualized, precision medicine for pediatric RMS will evolve, improving on the currently unacceptable survival rate," says Dr. Granberg.
For more information
Perez EA, et al. Rhabdomyosarcoma in children: A SEER population based study. Journal of Surgical Research. 2011;170:e243.
Crist WM, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. Journal of Clinical Oncology. 2001;19:3091.
Cytoreductive surgery and HIPEC offers effective treatment for selected patients with peritoneal carcinomatosis. Mayo Clinic.