May 13, 2023
In a clinical pilot study, an 11-MDM ovarian cancer panel, developed at Mayo Clinic, distinguished cancer and noncancer in plasma and identified all five early-stage, high-grade serous ovarian cancers included. Gynecologic Oncology published the research paper with this study's results in 2022.
Jamie N. Bakkum-Gamez, M.D., a Mayo Clinic gynecologic oncologist and study author, calls for larger studies with more-diverse patient populations to confirm this study's results. At the same time, she indicates she is pleased with the results thus far. This study's findings, while preliminary, encourage researchers like her who have been striving for an assay — especially an early detection assay — for ovarian cancer. At present, no effective early detection or screening tests for ovarian cancer exist.
The challenge of developing an early detection assay
For decades, researchers in ovarian cancer have sought a test that might allow physicians to catch ovarian cancer early and treat it at a more curable stage.
According to Dr. Bakkum-Gamez, many professionals in the ovarian cancer field anxiously awaited results of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, which lasted 20 years. This study, however, found no improved survival — a required outcome of the U.S. Preventive Services Task Force — or earlier recognition for ovarian cancer. The study randomized a participant group to transvaginal ultrasound plus a CA 125 blood test and a control group to usual care. There were no differences in clinical outcomes between the two groups.
Stage 2C ovarian cancer
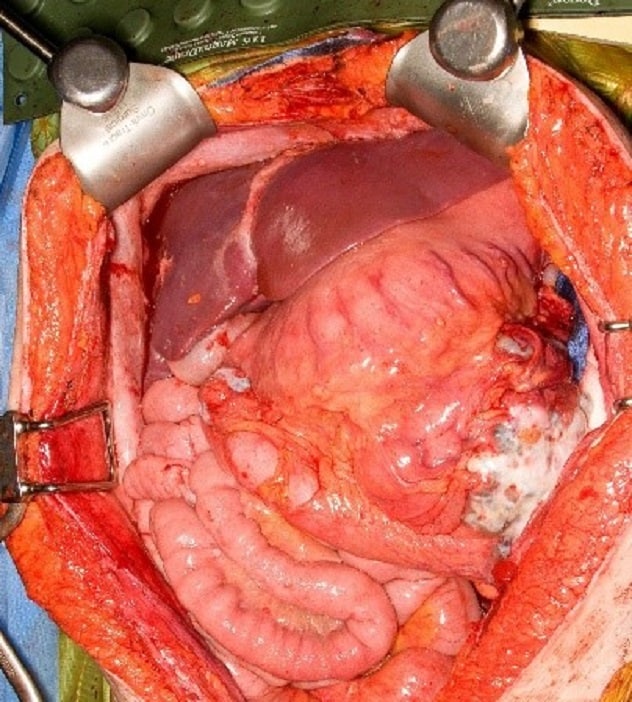
Stage 2C ovarian cancer
The white masses are omental caking.
Dr. Bakkum-Gamez's team leveraged the fact that alterations in DNA methylation, diverging from the norm, occur early in cancer formation. Thus, the team chose to pursue development of an assay making use of these changes to detect ovarian cancer in plasma. She explains that creating a marker panel presented challenges, however. One challenge was identifying patients with early-stage ovarian cancer to study at all because this cancer is not detected until stage 3 or 4 in approximately 75% of patients. However, a previously published mathematical modeling study suggests ovarian cancer can exist in a size less than 1 cm for months or even years, such that a window of time may exist in which small cancers might be detected.
Fallopian tube cancer

Fallopian tube cancer
Fallopian tube cancer (also high-grade serous cancer) involves the distal left fallopian tube.
"Additionally, many high-grade serous ovarian cancers — most of what we see in patients — start as small cancers, called serous tubal intraepithelial carcinomas (STICs), in the fallopian tube. It's like trying to find a needle in a haystack."
Consequently, the researchers continue to encounter challenges in identifying a biomarker panel that can detect ovarian cancer at the earliest stage and smallest size, she says, indicating that a high-sensitivity panel was critical.
The team performed reduced representation bisulfite sequencing to pinpoint ovarian cancer MDMs from DNA extracted from fresh frozen ovarian cancer tissue. The investigators compared these to DNA methylation within noncancerous fallopian tube epithelium and buffy coats from women who were cancer-free, who served as controls. The team selected MDMs by methylation fold change and receiver operating characteristics, plus low background methylation for the controls.
Pelvic ultrasound
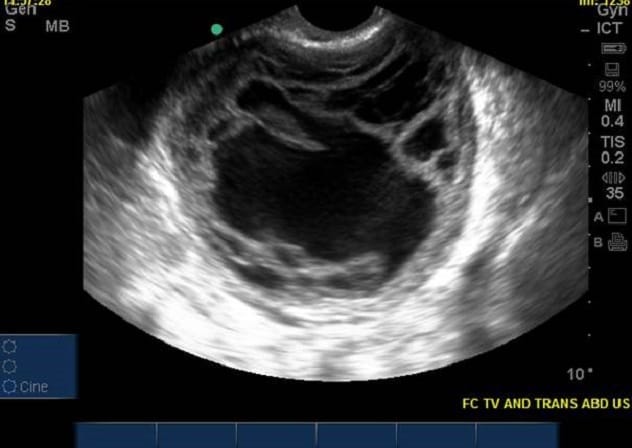
Pelvic ultrasound
Pelvic ultrasound shows a complex ovarian mass that was diagnosed as ovarian cancer at surgery.
The investigators then used methylated-specific polymerase chain reaction on DNA from independent ovarian cancer and fallopian tube epithelium formalin-fixed and paraffin-embedded tissue to perform a blinded biological validation. They employed Target Enrichment Long-Probe Quantitative-Amplified Signal assays on DNA from plasma from patients newly diagnosed with ovarian cancer prior to treatment and women with no ovarian cancer from the population to evaluate the ovarian cancer MDMs' performance. To determine predictive probability of ovarian cancer, they ran a random forest modeling analysis. The results were five hundredfold in silico cross-validated.
In the tissue-based marker discovery and validation, the researchers identified 33 MDMs with unique and marked methylation fold changes when considering all ovarian cancer subtypes versus benign fallopian tube epithelium. They moved 11 of these MDMs into a clinical pilot, testing plasma from 91 women with ovarian cancer, including 73 women with high-grade serous type and 91 women with no ovarian cancer diagnosis. The 11-MDM panel clearly distinguished ovarian cancer from controls, with 96% specificity and 79% sensitivity. The area under the receiver operating characteristic curve was 0.91. The panel precisely identified each of the five early-stage, high-grade serous ovarian cancers assessed.
The need for early ovarian cancer detection
The lack of early detection for ovarian cancer prompts this line of research as it is common for patients with ovarian cancer to have vague symptoms even when the cancer is stage 3 or 4. This late stage at diagnosis occurs in about 75% of women diagnosed with this cancer.
At this juncture of more-advanced disease, cure is often elusive. Patients may easily brush aside the symptoms or attribute symptoms to benign abdominal or gastrointestinal conditions, according to Dr. Bakkum-Gamez. Symptoms may include:
- Early satiety.
- Bloating.
- Bowel changes, such as new diarrhea or constipation.
- Bladder changes in frequency or urgency.
Dr. Bakkum-Gamez indicates a typical scenario for a woman diagnosed with ovarian cancer might look like the following: "A woman begins to experience an annoying discomfort with her clothes getting tighter. She wonders if this might be due to menopause or diet changes. Perhaps she gets diagnosed with IBS. It may be a long time. Statistics say the average time from onset of ovarian cancer symptoms till diagnosis is six months."
Some patients may have just one symptom, says Dr. Bakkum-Gamez, or have advanced disease and subtle symptoms. She says this is why ovarian cancer is often dubbed "the silent killer." Dr. Bakkum-Gamez notes she hopes the research she and the team are doing will ultimately lead to a test that can identify this potentially deadly disease when it is still early stage, such that it can be treated with improved survival outcomes.
For more information
Marinelli LM. Methylated DNA markers for plasma detection of ovarian cancer: Discovery, validation, and clinical feasibility. Gynecologic Oncology. 2022;165:568.
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO). National Cancer Institute, Division of Cancer Prevention.
Refer a patient to Mayo Clinic.
Financial disclosures
John B. Kisiel, M.D., Jamie N. Bakkum-Gamez, M.D., Douglas (Doug) W. Mahoney, M.S., and William R. Taylor, M.S., are inventors of Mayo Clinic intellectual property, which is licensed to Exact Sciences (Madison, Wisconsin), and may receive royalties, paid to Mayo Clinic.