May 22, 2018
The incidence of type 2 diabetes mellitus (T2DM) has reached an epidemic proportion of the human population. Recent estimates put worldwide prevalence of T2DM at 415 million. That number is expected to rise to 615 million by year 2040. This widespread emergence of T2DM presents one of the greatest challenges to global human health in this century. For this reason, understanding the molecular and physiological mechanisms underlying increased susceptibility to T2DM is an essential task for development of novel preventive and therapeutic approaches.
Aleksey Matveyenko, Ph.D., with Physiology and Biomedical Engineering at Mayo Clinic's campus in Rochester, Minnesota, says: "T2DM is a complex polygenic disease the pathophysiology of which involves interactions between genetic, epigenetic and environmental risk factors. Although genetic susceptibilities clearly play an important role in predisposition to T2DM, environmental factors appear to be significantly greater predictors of diabetes onset and progression.
"Indeed, environmental factors such as caloric intake and physical inactivity have long been appreciated to augment susceptibility to T2DM. More recently, however, circadian rhythm disruption has been gaining greater appreciation as an emerging environmental risk factor for T2DM."
Circadian disruption is defined as "misalignment between the endogenous circadian system and behavioral circadian cycles" (for example, sleep-wake and fasting-feeding). In today's 24-hour society, circadian disruption is becoming increasingly commonplace — driven primarily by increased exposure to artificial lighting, rotational and night shift work, social jet lag as well as comorbidities such as obesity and sleep disorders. For example, in the United States alone, more than 70 percent of adults report inadequate sleep quality and duration and nearly 20 million people are exposed to daily shift work-like conditions.
Dr. Matveyenko explains: "Multiple strands of evidence support the causative relationship between circadian disruption and impaired glucose homeostasis. First, individuals engaged in work conditions characterized by circadian disruption, such as rotational shift and night work, exhibit higher prevalence of diabetes, impaired glucose tolerance and metabolic syndrome.
"In addition, clinical studies performed under controlled laboratory settings show that acute exposure to circadian disruption results in dysregulation of glucose metabolism characterized by impaired insulin secretion and insulin action. Finally, additional support for the role of the circadian system in glucose homeostasis comes from genome-wide association studies showing an association between common genetic variants in key circadian-controlled genes such as CRY2 and MTNR1B and increased prevalence of hyperglycemia and T2DM.
Human circadian system as a multilevel oscillator network
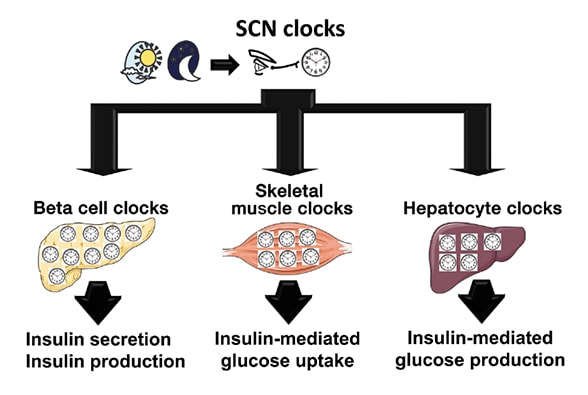
Human circadian system as a multilevel oscillator network
The human circadian system is organized as a multilevel oscillator network. The master circadian clock (pacemaker) is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, where it receives photic information from the ganglion cells in the retina. The process synchronizes the SCN clock to the solar day. The SCN subsequently integrates and synchronizes peripheral circadian clocks in metabolically active tissues, such as pancreatic beta cells, skeletal myocytes and hepatocytes, to the solar day by employing a combination of neuronal, behavioral and endocrine outputs. Subsequently, intracellular circadian clocks in metabolic tissues exert physiological control over glucose metabolism through regulation of insulin secretion (beta cell), insulin-mediated glucose uptake (skeletal muscle) and insulin-mediated hepatic glucose production (hepatocytes).
"To gain insights into mechanisms underlying circadian control of glucose metabolism, it is first important to review physiological regulation of the circadian system. The circadian system is organized as a multilevel oscillator network. The master circadian clock (pacemaker) is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN comprises molecular oscillators (also called circadian clocks), operating within individual neurons, governed by a precise transcriptional-translational feedback loop consisting of a set of core clock genes such as CLOCK and BMAL1.
"Importantly, autonomous circadian clocks are also present in numerous tissues outside of the SCN, including cell types essential for regulation of glucose metabolism, such as pancreatic beta cells, skeletal myocytes and hepatocytes. The SCN integrates and synchronizes peripheral circadian clocks to the solar day by employing a combination of neuronal, behavioral and endocrine outputs."
Dr. Matveyenko continues: "Recently, an increased emphasis has been placed on understanding how intracellular circadian clocks in metabolic tissues exert physiological control over glucose metabolism, and specifically, insulin secretion and insulin action. Circadian regulation of insulin secretion is particularly critical for normal regulation of beta cell function, given its significance to restrain insulin secretion during the inactive (sleep) phase, and optimize insulin production and release during the active (feeding) phase of the circadian cycle.
"For example, studies show that pancreatic beta cell circadian clocks regulate time-dependent transcription of key genes and transcription factors regulating beta cell glucose metabolism, oxidative stress, proliferation and insulin exocytosis. Subsequently, disruption of circadian clock function in beta cells results in impaired insulin secretory function, altered rate of cell proliferation and survival, and increased susceptibility for development of T2DM."
In addition to exerting control over insulin secretion, circadian clocks also regulate insulin action (or insulin sensitivity) through molecular control of postprandial glucose disposal and hepatic glucose production. Insulin-stimulated glucose uptake into skeletal muscle accounts for nearly 70 percent of the postprandial glucose clearance. This process is mediated through insulin-stimulated recruitment of GLUT4 glucose transporters to the plasma membrane, thus facilitating skeletal muscle glucose uptake and oxidation.
This process has been recently shown to be controlled by the skeletal muscle circadian clock, which ensures time-dependent expression and translocation of GLUT4 transporters to anticipate meal-induced glucose excursions. In addition, recent mouse genetic studies demonstrate that ablation of the circadian clock in hepatocytes disrupts hepatic glucose and lipid metabolism and consequently impairs normal regulation of insulin-mediated suppression of hepatic glucose production.
Dr. Matveyenko concludes: "Taken together, circadian rhythms in humans are regulated by a complex multilevel circadian oscillator system that undoubtedly provides an advantage for human health and is essential for maintaining proper metabolic control. However, this system becomes disadvantageous when lifestyle factors impose time constraints that produce circadian disruption — misalignment between internal circadian oscillators and the external environment.
"In this regard, understanding the molecular and physiological mechanisms responsible for circadian disruption-associated risk of T2DM warrants further research, and holds a potential for contributing to the development of novel therapeutic and preventive strategies for patients with T2DM."