Feb. 20, 2018
Normal enterohepatic circulation of bile acids
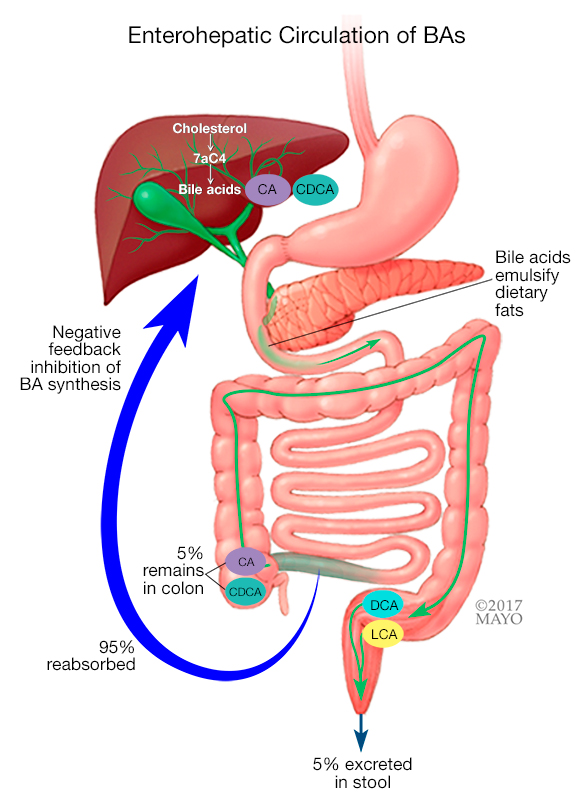
Normal enterohepatic circulation of bile acids
In cases of bile acid malabsorption, bile acids are ineffectively reabsorbed in the terminal ileum, which stimulates bile acid synthesis in the liver, resulting in increased concentrations of both serum 7αC4 and fecal bile acids.
Excess bile acids entering the colon can cause the classic signs and symptoms of bile acid malabsorption (BAM), including watery stool, urgency and fecal incontinence. Although BAM has been associated with diarrhea for nearly 50 years, it remains an underrecognized and underdiagnosed cause of chronic diarrhea.
Studies have demonstrated that BAM occurs in about one-third of patients diagnosed with irritable bowel syndrome with diarrhea (IBS-D), up to 50 percent of those with functional diarrhea and 35 percent of those with microscopic colitis.
Even so, BAM is seldom considered in most cases of chronic diarrhea encountered by gastroenterologists and primary care physicians. As a result, patients may be extensively investigated with colonoscopies, CT enterography and other stool studies, diagnosed with other causes of diarrhea, or considered to have irritable bowel syndrome or functional diarrhea by exclusion, thereby delaying specific treatment.
Testing for BAM in clinical practice
Michael Camilleri, M.D., a gastroenterologist at Mayo Clinic's campus in Minnesota, says that historically, evaluating patients for BAM has been the limited by the availability of accurate diagnostic tests.
"Bile acid diarrhea affects 1 or 2 percent of people in the community, which is the same approximate prevalence of celiac disease in the United States. However, there have been no readily available methods in the United States that directly measure it. The 75selenium homotaurocholic acid seven-day retention test (SeHCAT), which is validated and used in most European countries, is not available in the United States. The alternative has been a therapeutic trial of a bile sequestrant. But some of these drugs are poorly tolerated, especially resin formulations such as cholestyramine, the response is variable and their use is difficult to justify without a definitive diagnosis," he explains. "Today, however, there are two tests available in the U.S. for evaluating BAM — the serum 7αC4 test and the fecal bile acid test."
Fecal bile acid test
The first test option is the fecal bile acid excretion test. It quantifies individual and total bile acids in a 48-hour stool collection. Leslie J. Donato, Ph.D., co-director for Cardiovascular Laboratory Medicine, Hospital Clinical Laboratory and Point of Care Testing at Mayo Clinic's campus in Minnesota, says increased total fecal bile acids are seen in patients with chronic functional diarrhea, and higher levels of the primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA) are associated with IBS-D.
A clinical validation conducted at Mayo Clinic involving 94 healthy volunteers, 60 patients with IBS-D and 28 patients with IBS with constipation (IBS-C) found that the sum of CA and CDCA concentrations above 3.7 percent of the total fecal bile acids were indicative of IBS-D, with 72 percent sensitivity and 90 percent specificity. In addition, the upper limit of normal total fecal bile acid excretion over the 48 hours has been defined by collaborative work in the labs headed by Drs. Camilleri and Donato. So results of either total bile acids above the reference interval or CA+CDCA above 3.7 percent are consistent with BAM.
"The chemical measurement of fecal bile acids involves several steps of extraction, separation and quantification," Dr. Donato explains. "Essentially, the stool specimen is homogenized with water and bile acids are extracted from the specimen to remove proteins, lipids, salts and solid materials. Quantification is accomplished using high-performance liquid chromatography and mass spectrometry, which is extremely accurate."
At Mayo Clinic, the same 48-hour stool sample can be used to measure bile acids and fecal fat — an advantage for the evaluation of diarrhea of unknown etiology. Patients take the stool collection kit home, without the need for a visit to a tertiary care center. Test preparation includes a diet containing 100 grams of fat for two days prior to collection and during the 48-hour collection period. Samples must be kept frozen to preserve bile acid stability. The 48-hour collection is then mailed to the laboratory for analysis.
The fecal bile acid excretion test will likely have multiple uses, including evaluation of patients with Crohn's disease who have diarrhea after ileal resection or ileal inflammatory disease that impedes bile acid absorption in the distal ileum (including radiation and Crohn's enteritis) and those with post-cholecystectomy diarrhea.
The fecal bile acid test is available to physicians within and outside Mayo through Mayo Medical Laboratories (test ID: BA48F).
Serum 7αC4 test
The second test option for BAM is the 7αC4 test, which measures fasting serum levels of the marker 7α-hydroxy-4-cholesten-3-one (abbreviated to 7αC4 or C4, but do not confuse it with serum complement C4 test), a downstream product of CYP7A1. Fasting serum C4 levels increase when bile acid synthesis increases, and C4 levels are substantially elevated in BAM patients. C4 levels have also been shown to correlate well with SeHCAT seven-day retention and fecal bile acids. With the ease of sample collection compared with fecal bile acids, this makes fasting serum C4 attractive as a screening test for BAM, although it can produce false-positives and false-negatives in patients who have liver disease or are taking statins.
Interpretive guide for BAM screening test results

Interpretive guide for BAM screening test results
Bile acid malabsorption (BAM) interpretive guide
Internal studies have shown that a C4 concentration of 17.6 ng/mL maximizes sensitivity while a result of 52.5 ng/mL maximizes specificity. An interpretive guide is available for additional clarification.
"Serum C4 test was not developed at Mayo, but our staff validated and refined the assays, proving that it's useful, and we now offer it as an orderable test," Dr. Camilleri says. An article describing Mayo's development of a serum C4 assay appeared in the March 2009 issue of Neurogastroenterology & Motility, and further validation and clinical utility appeared online in Clinical Biochemistry in October 2017.
The serum 7αC4 test is available to physicians within and outside Mayo through Mayo Medical Laboratories (test ID: 7AC4).
Clinical impact
"We've conclusively demonstrated that about a third of patients with chronic diarrhea thought to be due to IBS-D or functional diarrhea have BAM and need to be diagnosed properly and treated more specifically and effectively," Dr. Camilleri says. His comprehensive review of bile acid diarrhea appeared in the January 2014 issue of Expert Review of Gastroenterology & Hepatology.
For more information
Camilleri M, et al. Measurement of serum 7α-hydroxy-4-cholesten-3-one (or 7αC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterology & Motility. 2009;21:734.
Camilleri M. Advances in understanding of bile acid diarrhea. Expert Review of Gastroenterology & Hepatology. 2014;8:49.
Donato LJ, et al. Description of analytical method and clinical utility of measuring serum 7-alpha-hydroxy-4-cholesten-3-one (7aC4) by mass spectrometry. Clinical Biochemistry. In press.