Sept. 05, 2012
Graves' ophthalmopathy (GO) is an inflammatory autoimmune disorder of the orbit that primarily affects patients with a history of Graves' hyperthyroidism. However, it is also seen in euthyroid and hypothyroid individuals who have never been hyperthyroid.
Rebecca S. Bahn, M.D., of the Division of Endocrinology, Diabetes, Metabolism, & Nutrition at Mayo Clinic in Rochester, Minn., explains: "It's clear that autoantibodies directed against the thyrotropin (TSH) receptor, termed TRAb, cause Graves' hyperthyroidism by stimulating the thyroid to produce excess thyroid hormones. TRAb can be detected using sensitive assay systems in essentially all patients with GO, including those without a history of hyperthyroidism. Levels of TRAb correlate with the severity and inflammatory activity of GO, and the high titers of these antibodies predict a worse prognosis.
"Although the onset of GO occasionally precedes or follows the onset of hyperthyroidism by many years, these conditions most commonly are diagnosed simultaneously or within about 18 months of each other. Because of the close clinical relation between Graves' hyperthyroidism and GO, investigators have long hypothesized that both autoimmune conditions derive from a single systemic process and share the TSH receptor as a common autoantigen."
Dr. Bahn continues: "Our laboratory is interested in unraveling the pathogenesis of GO because a better understanding of the disease will enable development of novel and improved forms of therapy for this debilitating condition. The GO-affected orbit is characterized by edema, hyaluronic acid (HA) accumulation, and increased volume of adipose tissue and extraocular muscles. The adipose tissue enlargement is in part due to the development of new fat cells within the orbital tissues. The muscle enlargement is produced as hydrophilic HA, and edema collects within the connective tissues lying between the intact muscle fibers. The increase in tissue volume within the bony orbit displaces the globe forward and hinders venous outflow. As a result, cytokines and other mediators of inflammation, produced by infiltrating mononuclear cells and resident macrophages, accumulate within the orbit and contribute to the local inflammatory process."
Dr. Bahn further reports: "We have shown that TSH receptor is highly expressed in GO orbital tissues and is found specifically on the resident fibroblasts. Higher levels of TSH receptor expression can be measured in orbital fibroblasts from GO patients than in fibroblasts from normal orbits or other parts of the body. Although some orbital fibroblasts are adipocyte precursor cells that further increase TSH receptor expression as they differentiate into mature adipocytes, others are capable of producing HA in large quantities. These and other findings suggest that orbital fibroblasts are the target cells in GO and that stimulation of TSH receptor on these cells by circulating TRAbs may contribute to the tissue remodeling characteristic of the disease."
New fat cell development
The role of the thyrotropin (TSH) receptor in the immunopathogenesis of GO
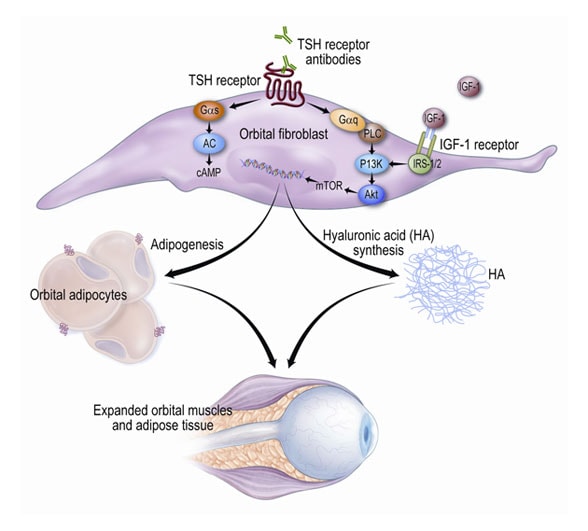
The role of the thyrotropin (TSH) receptor in the immunopathogenesis of GO
Circulating TSH receptor stimulatory autoantibodies recognize the receptor on fibroblasts residing within the orbit. Ligation of the receptor results in activation of the phosphoinositide-3-kinase (P13K)/Akt signaling cascade, as well as others, including the adenylyl cyclase (AC)/cAMP pathway. This leads to increased production of hyaluronic acid (HA) by these cells and the development of new fat cells from a subset of adipocyte precursors. In addition, insulinlike growth factor (IGF-I) and other growth factors and cytokines within the orbit may modulate these processes. This results in accumulation of HA and edema within the orbit and expansion of the orbital fat volume, leading to the varied clinical expressions of the disease. Iyer S, et al. Immunopathogenesis of Graves' ophthalmopathy: The role of the TSH receptor (http://www.bprcem.com/article/S1521-690X(11)00130-8/abstract). Best Practice & Research. Clinical Endocrinology & Metabolism. 2012; 26(3):281-289. Used with permission.
An important question is whether TSH receptor activation by TRAbs might impact new fat cell development. To address this question, Dr. Bahn says: "We treated GO fibroblasts with a high-affinity human stimulatory monoclonal antibody directed against TSH receptor (termed M22). We found that M22 stimulates not only cyclic adenosine monophosphate (cAMP) production in orbital fibroblasts (as it does in thyrocytes), but it also activates phosphoinositol-3-kinase (P13K) pAkt/mTOR signaling. In doing so, it acts as a pro-adipogenic factor to increase expression of genes found in late stages of adipogenesis and promotes lipid accumulation within the cells.
"This action was reversed when inhibitors of this pathway were introduced into the laboratory cultures. Similarly, insulinlike growth factor 1 (IGF-I), which is present in high levels within the GO orbit, acts to stimulate adipose cell development in orbital preadipocyte fibroblasts. In other studies, we showed that M22, other stimulatory TRAbs, and IGF-I also increase HA synthesis in GO orbital fibroblasts. These findings help to explain the orbital tissue changes characteristic of GO. In addition, they suggest that inhibition of TSH receptor signaling might block disease-related effects of TRAbs on orbital fibroblasts and may thus represent a novel approach to therapy."
Drs. Susanne Neumann and Marvin C. Gershengorn of the Clinical Endocrinology Branch, National Institutes of Health, in Bethesda, Md., have recently developed low-molecular-weight antagonists of thyrotropin- and TRAb-stimulated TSH receptor signaling. Dr. Bahn explains: "These compounds, modeled after similar antagonists of luteinizing hormone and follicle-stimulating hormone, were developed using high-throughput screening and functional experiments. Acting as allosteric modulators, they sit within the transmembrane portion of TSH receptor and prevent activation of the receptor without interfering with TSH or TRAb binding to the receptor.
"In studies using human thyrocytes, these compounds have been shown to inhibit cAMP production stimulated by immunoglobulins from the sera of patients with Graves' hyperthyroidism. Because these small drug-like compounds are not degraded in the gastrointestinal tract, they carry potential as oral agents for the treatment of both Graves' hyperthyroidism and GO.
"We are currently collaborating with Drs. Neumann and Gershengorn to study these TSH receptor antagonists in our tissue culture model of GO. Our early studies have revealed a 50 to 70 percent decrease in cAMP production, Akt phosphorylation, and HA synthesis in cells treated with both a stimulatory TRAb and a TSH receptor antagonist, compared with cultures containing the TRAb alone."
Dr. Bahn summarizes: "GO is a debilitating ocular disease for which no uniformly effective therapy exists at present. Recent insights into the important role of TSH receptor and TRAb in the development of the tissue remodeling characteristic of the disease suggest new approaches to therapy. Rather than focusing primarily on suppression of the autoimmune process itself, we are directing efforts toward understanding the impact of small drug-like TSH receptor antagonists on orbital fibroblast functions relevant to disease development. These compounds hold promise not only because they are novel for established GO, but also because they could pertain to both the treatment of hyperthyroidism and the prevention of ocular changes in patients with Graves' disease."