May 20, 2015
Nearly half of all patients with heart failure have a normal ejection fraction (EF). The prevalence of this syndrome, termed heart failure with preserved ejection fraction (HFpEF), continues to increase in the developed world, likely because of the increasing prevalence of common risk factors, including older age, female sex, hypertension, metabolic syndrome, renal dysfunction and obesity.
Interaction of factors resulting in symptomatic HFpEF
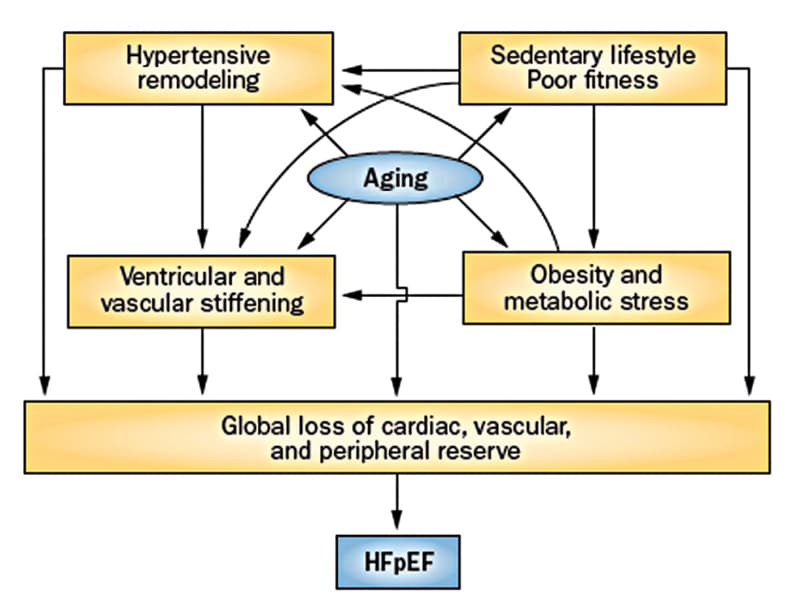
Interaction of factors resulting in symptomatic HFpEF
The interaction between risk factors, cardiac aging and loss of cardiovascular reserve, which results in the development of symptomatic HFpEF. Based on Nature Reviews Cardiology. 2014;11:507. Used with permission.
Hypertension in particular is a strong risk factor; 80 to 90 percent of patients with HFpEF are hypertensive. Historically, HFpEF was termed diastolic heart failure; however, recent investigations suggest a more complex and heterogeneous pathophysiology. Ventricular diastolic and systolic reserve abnormalities, chronotropic incompetence, stiffening of ventricular tissue, atrial dysfunction, pulmonary hypertension, impaired vasodilation, and endothelial dysfunction are all implicated. Frequently, these abnormalities are noted only when the circulatory system is stressed.
Pathophysiology
At a cellular level, cardiac myocytes in patients with HFpEF are thicker and shorter than normal myocytes, and collagen content is increased. Recent histologic studies have shown reductions in myocardial capillary density that may contribute. At the organ level, affected individuals may have concentric remodeling with or without hypertrophy, although many people have normal ventricular geometry. Increases in myocyte stiffness are mediated in part by relative hypophosphorylation of the sarcomeric molecule titin, due to cyclic guanosine monophosphate (cGMP) deficiency thought to arise primarily as a consequence of increased nitroso-oxidative stress induced by comorbid conditions such as obesity, metabolic syndrome and aging. Cellular and tissue characteristics may become more pronounced as the disease progresses.
"Most studies suggest that the rate of left ventricular (LV) pressure decay during isovolumic relaxation is prolonged, increasing LV and left atrial (LA) pressure, especially with elevated heart rates, as during exercise," says Barry A. Borlaug, M.D., a cardiologist at Mayo Clinic in Rochester, Minnesota.
Normal ventricular filling is achieved in large part by ventricular suction, the early active component of diastole, which is generated by:
- Intraventricular pressure gradients
- Mitral annular longitudinal motion
- Early diastolic LV "untwisting"
- Elastic recoil induced by contraction to a smaller end systolic volume in the preceding contraction cycle
Each of these four elements is impaired in patients with HFpEF, especially with stress, so filling becomes dependent on high LA pressure to actively push blood into the left ventricle, as opposed to the action of a normal left ventricle, which "pulls" blood in during early diastole. Passive LV end-diastolic stiffness (Eed) is quantified by the slope and position of the diastolic pressure-volume relationship. Eed increases with normal aging, but this increase is exaggerated in individuals with HFpEF in most, but not all studies.
Although systolic function is relatively preserved, individuals with HFpEF typically exhibit subtle abnormalities in systolic performance, which become more dramatic during exercise. Limited stroke volume reserve and chronotropic incompetence markedly limit cardiac output in response to exercise. Mechanical dyssynchrony is common even though electrical dyssynchrony is not. Atrial fibrillation is extremely common in HFpEF (seen at some point in two-thirds of patients) and poorly tolerated because of the importance of LA contractile function in maintaining adequate LV chamber filling.
Pulmonary hypertension is common in patients with HFpEF. Increased LA pressure adds in series with increased resistive and pulsatile pulmonary arterial loading to increase RV afterload. This then leads to RV dysfunction, which seems to be tightly correlated with the development of atrial fibrillation. With RV failure, progressive systemic congestion occurs, manifested by malabsorption, congestive hepatopathy, cardiorenal syndrome, systemic inflammation and cardiac cachexia.
Increased RV and LA size and subsequent increases in total cardiac volume can lead to pericardial restraint, preventing additional preload recruitment during exercise or saline loading and contributing to elevation in filling pressures and cardiac output plateau.
Diagnosis
The most common complaints at presentation are exertional dyspnea and fatigue. Currently, three criteria must be met to establish the diagnosis:
- Clinical symptoms consistent with heart failure
- Preserved EF (at least 50 percent)
- Evidence of cardiac dysfunction
"Objective evidence of cardiac dysfunction is the most controversial point and a topic of intense investigation," says Dr. Borlaug. Potential findings that demonstrate cardiac dysfunction include evidence of congestion on physical examination or chest X-ray; atrial fibrillation; echocardiographic evidence of diastolic dysfunction (LA enlargement, engorged inferior vena cava, pulmonary hypertension or elevated E/e´ filling velocity); and elevated brain natriuretic peptide (BNP).
If the diagnosis remains uncertain after testing, invasive hemodynamic evaluation may be revealing. Elevated filling pressures at rest support the diagnosis of HFpEF; however, many individuals demonstrate hemodynamic compromise only with stress. In these patients, it is critical to perform hemodynamic measurements during exercise.
Treatment
Comparison of clinical characteristics and risk factors in recent larger clinical trials
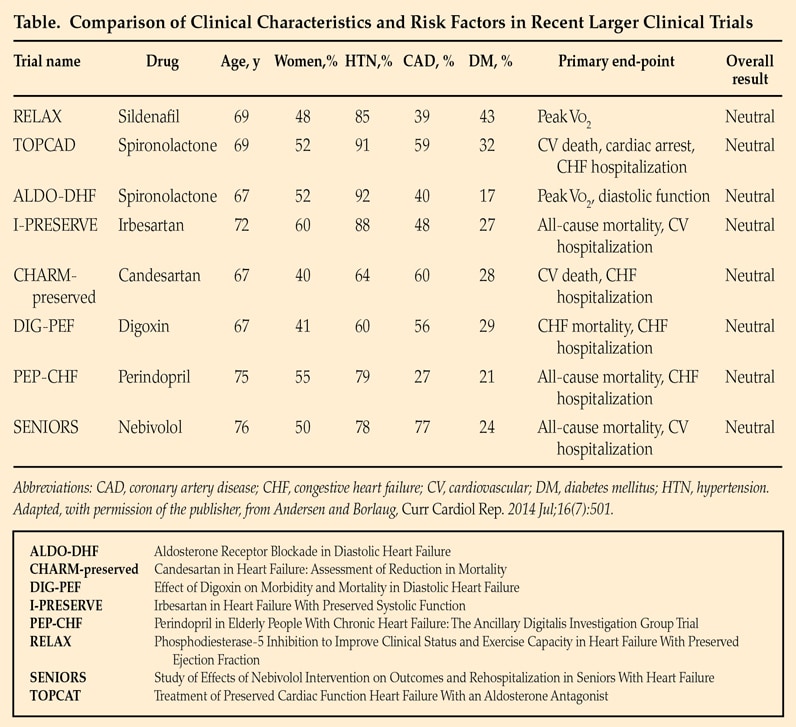
Comparison of clinical characteristics and risk factors in recent larger clinical trials
Clinical trials have not yet identified effective treatments for HfpEF. Recommendations are based on expert consensus opinion and focus on treatment of hypertension and maintaining appropriate intravascular volume. Based on Current Cardiology Reports. 2014;16:501. Used with permission.
Clinical trials have not yet identified effective treatments for HFpEF. An article published in Current Cardiology Reports in 2014 compares clinical characteristics and risk factors in recent larger clinical trials.
Thus, recommendations are based on expert consensus opinion and focus on treatment of hypertension and maintaining appropriate intravascular volume. Aldosterone antagonists were not beneficial in a large multicenter trial overall, but a subgroup analysis of patients enrolled in the Americas showed some benefit on the basis of an elevated BNP level. Because HFpEF is associated with reduced cGMP availability, it was thought that treatment with phosphodiesterase-5 inhibitors, which increase cGMP levels via reduced metabolism, might be helpful. Unfortunately, the multicenter RELAX trial found no benefit for sildenafil compared with placebo in terms of exercise capacity, cardiac function or clinical status.
Many treatment aspects have not yet been evaluated in a controlled fashion. Although atrial fibrillation is very common in patients with HFpEF, it is not known whether a rate or rhythm control strategy is preferential. It is also not known whether aggressive revascularization in those individuals with both coronary artery disease and HFpEF affects outcomes, although a recent single-center observational study showed improved survival in patients receiving complete revascularization. Some studies have also shown a beneficial response to statins in HFpEF, although this has not been evaluated in this population in a pivotal trial.
Incomplete understanding of the pathophysiology of HFpEF, the likelihood that there is substantial pathophysiologic heterogeneity among affected patients, and the interplay of various risk factors have all been barriers in the development of effective treatments, underscoring the need for expanded research initiatives, given the rapidly increasing number of patients with this form of cardiac failure.
Clinical trials in HFpEF at Mayo Clinic
Efficacy study of pacemakers to treat slow heart rate in patients with heart failure. Mayo Clinic.
Mayo Clinic. Inhaled sodium nitrite on heart failure with preserved ejection fraction. ClinicalTrials.gov.
Contact Barry A. Borlaug, M.D., principal investigator, at borlaug.barry@mayo.edu or Katlyn E. Koepp, study coordinator, at Koepp.Katlyn@mayo.edu or 507-255-2200.
For more information
Anderson MJ, et al. Heart failure with preserved ejection fraction: Current understandings and challenges. Current Cardiology Reports. 2014;16:501.
Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nature Reviews Cardiology. 2014;11:507.