May 04, 2018
Atrial fibrillation (AF) remains the most common significant cardiac arrhythmia in the United States, with a prevalence estimated to be approximately 7 million patients and predicted to increase to 16 million by 2050. This arrhythmia may occur in patients with no associated cardiovascular disease (sometimes termed lone AF), or more commonly with a variety of underlying cardiac conditions. The prevalence increases with age so that 25 percent of patients older than 45 years of age have experienced one or another of the types of AF.
Symptoms related to AF vary widely; the predominant concern has been the relationship between AF and stroke, combined with the observation that both AF and stroke increase with advancing age. It has been estimated that AF accounts for 20 to 30 percent of all stroke in patients over 75 years of age.
In patients with nonvalvular AF, specifically defined as patients without mitral valve obstruction to flow, studies have identified that the left atrial appendage is the source of cardioembolic stroke in approximately 90 percent of patients. "These cardioembolic strokes are associated with the highest morbidity and mortality as well as an increased rate of recurrence and hemorrhagic transformation," according to David R. Holmes Jr., M.D., an interventional cardiologist at Mayo Clinic's campus in Rochester, Minnesota.
For the prevention of cardioembolic strokes, anticoagulation has been the mainstay of therapy. Initially, warfarin was the sole agent available and was found to reduce the incidence of stroke in the setting of AF by approximately 60 percent. However, warfarin administration is complicated due to individual variability in dosing, need for frequent monitoring and dosage adjustment, drug-drug interactions, and bleeding hazards.
Direct oral anticoagulants (DOACs) are increasingly used, and as a whole, they are more effective than warfarin in stroke prevention with a lower risk of intracerebral hemorrhage. Additional advantages include the lack of need for periodic monitoring of their effect and a set dosage (although dosing needs to be adjusted for some of the DOACs based upon renal function).
Despite the advantages, these agents have not been as widely adopted as predicted because of the incidence of gastrointestinal bleeding (which is similar or even slightly increased compared with warfarin), cost, inconvenience (some of the DOACs require twice-daily dosing) and lack of widely available reversal agents.
Despite the availability of multiple agents, approximately 40 percent of patients at risk of stroke are not treated with anticoagulation because of a relative or absolute contraindication such as prior intracerebral hemorrhage or frequent falls.
Finally, in large-scale studies, anticoagulation therapy is discontinued in 50 to 60 percent of patients within one year after initiation of treatment, and they remain at increased risk of stroke.
For all of these reasons, local site-specific therapy with mechanical closure of the left atrial appendage has received increasing attention. Multiple devices are available globally either in clinical trials or in development; however, only one device is currently approved in the United States (Watchman). The device was approved based on the results of two randomized clinical trials and two accompanying registries. Subsequently, it has been evaluated in a large international registry and a post-approval U.S. trial.
Procedural success in individual Watchman trials
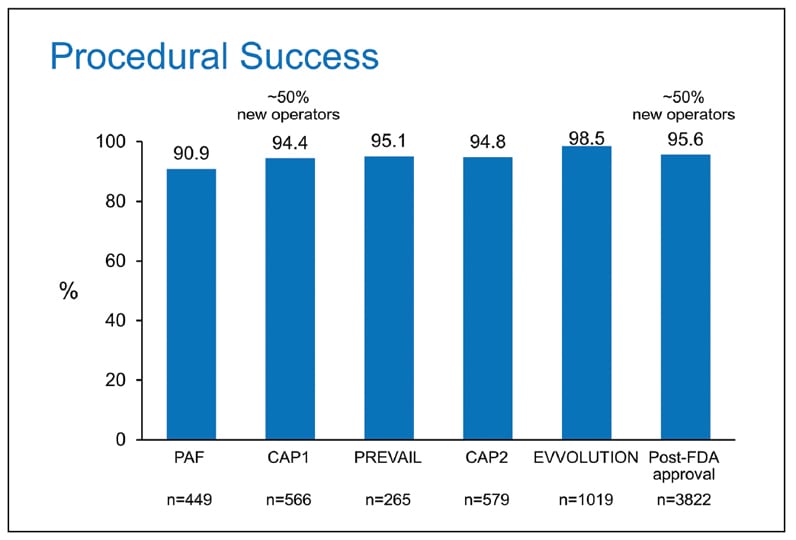
Procedural success in individual Watchman trials
Procedural success in individual Watchman trials defined as deployment and release of the device into the left atrial appendage and no leak greater than 6 mm.
Procedural complications in Watchman studies
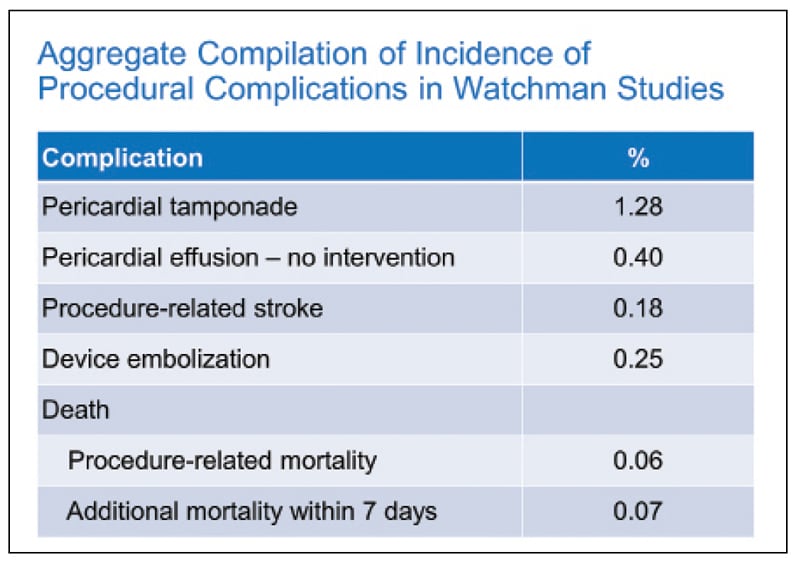
Procedural complications in Watchman studies
Aggregate compilation of procedural complications in Watchman studies.
Meta-analysis of patients receiving Watchman device versus warfarin
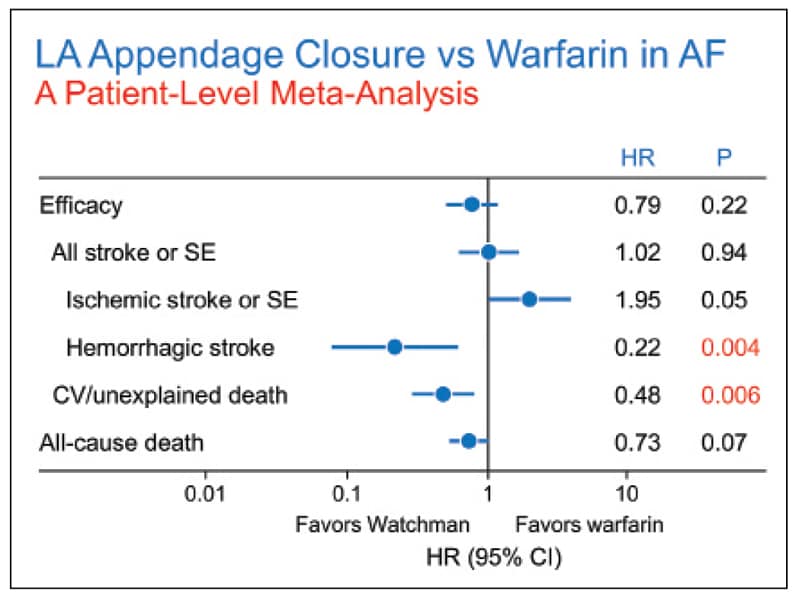
Meta-analysis of patients receiving Watchman device versus warfarin
Meta-analysis of patients receiving Watchman device versus warfarin for overall stroke, ischemic stroke, and all-cause death in PROTECT AF and PREVAIL trials. Reprinted with permission from the Journal of the American College of Cardiology.
In the largest patient meta-analysis comparing the two randomized trials, both of which evaluated device versus warfarin control, the overall composite of all-cause stroke or systemic embolism was similar; there was, however, an 80 percent reduction in hemorrhagic stroke. There was also marked 70 percent reduction in longer term bleeding in the device limb and a 50 percent reduction in cardiovascular or unexplained death. Procedural success has exceeded 90 percent in all trials, especially with experienced operators, and the incidence of complications has been low.
Indication for use (IFU) of the Watchman device is to reduce the risk of stroke or systemic thromboembolism from the left atrial appendage in patients with nonvalvular AF who:
- Are at increased risk of stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores and are recommended for anticoagulation therapy
- Are deemed by their physicians to be suitable for short-term warfarin
- Have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin
U.S. reimbursement status for this device was clarified in the CMS National Coverage Decision 2-8-16, which outlined criteria for coverage:
- CHADS2 score ≥ 2 or CHA2DS2-VASc score ≥ 3
- A formal shared decision-making interaction with an independent noninterventional physician using an evidence-based decision tool on oral anticoagulation in patients with nonvalvular AF
- Suitable for short-term warfarin but deemed unable to take long-term oral anticoagulation
Given this information, who should be considered for the Watchman device? At the present time it includes patients meeting the FDA IFU criteria:
- Nonvalvular AF at high risk of stroke (CHA2DS2-VASc score ≥ 3)
- Deemed suitable to take an anticoagulant for six weeks post implant (for facilitative endothelialization) following which they can be treated with anti-platelet agents alone
- Not considered good candidates for long-term anticoagulation
In patients in whom anticoagulation is felt to be contraindicated, there is a dilemma. At the present time there is an FDA trial randomizing patients to either Watchman plus aspirin versus a control condition of aspirin or aspirin plus Plavix. The results of this trial will have important implications for the field.
However, globally these devices are used without anticoagulation and instead patients are treated only with anti-platelet therapy. Accordingly, some patients in the U.S. with an absolute contraindication to any anticoagulation may be offered a Watchman device after very careful consideration of the alternatives by a team of physicians. It must be remembered that this is an off-label indication.
The steps involved in evaluating a Watchman candidate:
- Documentation of nonvalvular AF.
- Assessment of underlying comorbidities and risk assessment for stroke using the CHA2DS2-VASc score.
- Imaging of the left atrial appendage to evaluate other potential causes of stroke and exclude residual thrombus as well as documenting suitable anatomy for implantation. This can be performed with TEE either alone or in combination with CT.
- Careful evaluation and shared decision-making about risks and benefits of the procedure. This evaluation may be carried out by a variety of individuals in general cardiology, electrophysiology and neurology.
"Left atrial occlusion devices are not appropriate for every patient with AF. However, they are an option for those patients at significant risk of thromboembolic stroke for whom long-term anticoagulation is either contraindicated or felt to be suboptimal," says Dr. Holmes.
For more information
Holmes DR Jr, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: A patient-level meta-analysis. Journal of the American College of Cardiology. 2015;65:2614.