Aug. 26, 2023
Mayo Clinic is investigating stem cell treatment for intracerebral hemorrhage (ICH). The researchers hope that future clinical trials will lead to therapies that provide neuronal protection or recovery for people with ICH.
"This is cutting-edge research, using cells as drugs to help to repair the damage that occurs in hemorrhagic stroke," says Abba C. Zubair, M.D., Ph.D., a transfusion medicine and cell therapy specialist at Mayo Clinic in Jacksonville, Florida. "We haven't had treatment options that address neuronal recovery or protection of neurons. A regenerative therapy would be highly beneficial."
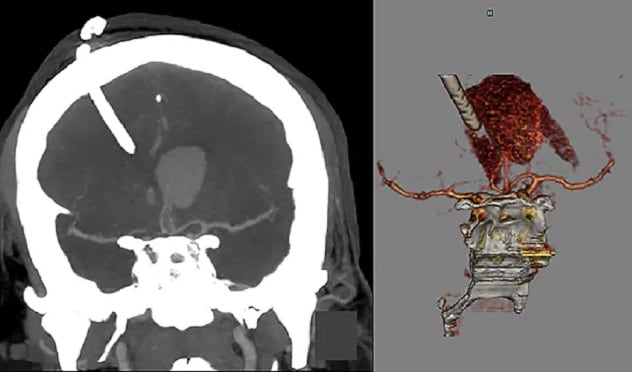 Intracerebral hemorrhage and external ventricular drain
Intracerebral hemorrhage and external ventricular drain
On the left, the CT image shows an intracerebral hemorrhage (ICH) with external ventricular drain (EVD) in the coronal plane. On the right, the drawing illustrates placement of an EVD in an ICH.
Roughly half of hemorrhagic strokes result in death within 30 days. Current treatment modalities focus on controlling inflammation and providing supportive therapies.
"Although significant advances have occurred in the treatment of stroke, the subset of patients with hemorrhagic stroke hasn't benefited as much. We are passionate about raising the level of treatment for ICH closer to our ability to treat patients with ischemic stroke," says William D. Freeman, M.D., a neurocritical care specialist at Mayo Clinic's Florida campus.
"This is cutting-edge research, using cells as drugs to help to repair the damage that occurs in hemorrhagic stroke."
The researchers have completed a phase 1 clinical trial targeting acute ICH treated within a week of stroke onset. "Our preclinical experiments showed that the effects of treatment aren't as prominent after delay. In the future, we would hope to study treatment that occurs later," Dr. Zubair says.
The phase 1 trial, for which Dr. Zubair served as co-principal investigator, involved three groups with three patients each. Each group received allogenic stem cells intravenously, at three different dose levels.
Results of the trial, conducted in conjunction with Mayo Clinic's Center for Regenerative Biotherapeutics, are being prepared for publication. Planning is underway for additional trials.
Possible mechanism of action
Mayo Clinic's preclinical studies demonstrated that mesenchymal stem cells have anti-inflammatory properties and the capacity to rescue injured neurons. In these experiments, neuronal cells were subjected to oxygen-glucose deprivation stress, which resulted in a significant decrease in cell proliferation and an increasing rate of apoptosis, as well as elevated levels of tumor necrosis factor-alpha. Subsequent co-culture of the neuronal cells with mesenchymal stem cells alleviated those effects.
The researchers believe that interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF), which are known to be secreted by mesenchymal stem cells, play an important role in stem cells' mechanism of action.
"Our laboratory work showed that when IL-6 or VEGF is blocked, the mesenchymal stem cells do not have the positive effects of regenerating neurons and improving their viability," Dr. Zubair says.
The preclinical experiments also demonstrated that mesenchymal stem cells don't need to be in direct contact with injured neurons to exert their regenerative effect — suggesting that stem cells can remotely affect the site of brain injury by releasing factors that permeate the blood-brain barrier. Indeed, while evaluating various methods of delivering stem cells, the researchers found that injection of stem cells directly into the stroke site was less effective than the intravenous or intraventricular approaches.
"We were surprised that direct injection doesn't seem to work," Dr. Zubair says. "But in our preclinical work, both intravenous and intraventricular approaches were effective."
The studies are the culmination of years of laboratory work. "One of our core values at Mayo Clinic is integrating basic science into the clinical practice," Dr. Freeman says. "We feel there can be rapid leaps in care to help patients with ICH. Our goal is to improve the welfare of those patients."
For more information
Clinical Trials: Mesenchymal Stem Cells Therapy in Patients With Recent Intracerebral Hemorrhage. Mayo Clinic.
Center for Regenerative Biotherapeutics. Mayo Clinic.
Refer a patient to Mayo Clinic.