April 18, 2020
Both acute diverticulitis and acute pancreatitis have sudden onset, involve visceral fat and affect individuals with similar demographics. Despite these similarities, patient outcomes associated with these diagnoses are quite different. Diverticulitis is often treatable in the outpatient setting and rarely causes organ failure, whereas acute pancreatitis requires hospitalization and has a higher risk of progression to multiple complications, including multisystem organ failure.
To examine the mechanisms underlying the different outcomes associated with these conditions, Mayo Clinic researchers recently conducted a study comparing the patterns of visceral adipose injury present during acute pancreatitis and acute diverticulitis. The results of this study were published in 2020 in the Journal of Clinical Investigation.
Study aims and methods
The researchers' primary aim was to learn why visceral adipose tissue involvement can worsen outcomes for patients with pancreatitis but not for those with diverticulitis. According to Vijay P. Singh, M.B.B.S., a gastroenterologist at Mayo Clinic's campus in Scottsdale, Arizona, and the study's principal investigator, the team began by examining the pathophysiology of nonesterified fatty acid (NEFA) generation in human acute diverticulitis and acute pancreatitis, and the roles of pancreatic lipases and adipocyte triglyceride lipase (ATGL) in the progression to organ failure. And they compared the pattern of fat necrosis that occurs after the onset of these conditions and analyzed visceral fat samples removed at the time of surgery for these diseases.
Using in vitro and in vivo models of acute pancreatitis, the team tested the efficacy of three approaches to preventing the cascade of lipolysis that can result in organ failure: pharmacologic approaches using the ATGL inhibitor (atglistatin) or the generic lipase inhibitor orlistat; genetic deletion of an adipocyte-specific ATGL; and genetic deletion of an enzyme called pancreatic triglyceride lipase (PNLIP), the major contributor to lipolytic activity in the pancreas.
Specially bred obese mice, including ATGL knockouts, PNLIP knockouts and control group littermates, were studied in mechanistically distinct severe pancreatitis models or injected with pancreatic lipases to examine how acute lipolysis of visceral adipose tissue and consequent local and systemic lipotoxic injury occur during acute pancreatitis.
Results and conclusions
Pancreatitis
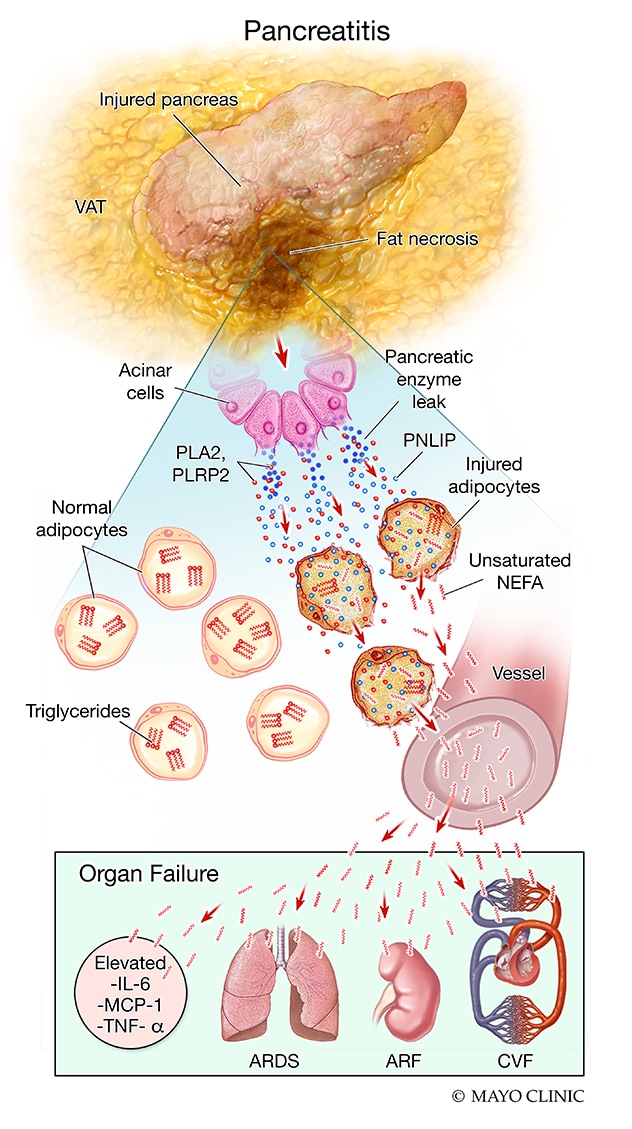
Pancreatitis
Progression of visceral adipose injury, rapid fat necrosis, worsening inflammation and organ failure that can occur during acute pancreatitis
Analysis of visceral fat samples removed at the time of surgery from patients in the acute pancreatitis group, which had significantly more organ failure than the group with diverticulitis, demonstrated that the fat cells were necrosed, and that chemically, the fat had broken down into fatty acids. The researchers also noted an increase in the amount and activity of pancreatic lipase in this necrosed fat. This suggested that PNLIP had leaked from the damaged pancreas into the necrosed fat and had perhaps generated NEFAs, which caused the organ failure. The researchers also noted that ATGL was absent in the fat taken from patients with pancreatitis but was present in samples taken from patients with diverticulitis.
After injecting pancreatic lipase into mouse visceral adipose tissue, the researchers learned that pancreatic lipase can hydrolyze adipose triglycerides and generate excess NEFAs, which can cause systemic inflammation and organ failure in the absence of acute pancreatitis. They also observed that PNLIP increased in adipose tissue during pancreatitis and entered adipocytes via multiple mechanisms, hydrolyzing adipose triglycerides and generating excessive NEFAs.
During pancreatitis, obese PNLIP knockout mice had lower visceral adipose tissue lipolysis, milder inflammation, less severe organ failure and improved survival compared with obese adipocyte-specific ATGL knockouts. Unlike ATGL knockouts, PNLIP knockout mice were protected from adipocyte-induced pancreatic acinar injury without effects on NEFA signaling or acute pancreatitis induction. This contrast suggests that during pancreatitis, but not diverticulitis, PNLIP leaks into visceral adipose tissue and can cause excessive visceral adipose tissue lipolysis independent of adipocyte-autonomous ATGL, thereby worsening organ failure.
Key points
- In humans, PNLIP is the primary mediator of the rapid fat degradation that occurs during pancreatitis.
- Pancreatic lipase hydrolyzes visceral adipose tissue, causing fat necrosis, organ failure and worsening inflammation.
- Drug-induced inhibition or genetic deletion of ATGL does not prevent damage caused by excess NEFA generation.
- PNLIP deficiency, but not ATGL deficiency, prevents breakdown of visceral fat during severe acute pancreatitis and improves outcomes.
- Reduction in PNLIP activity does not affect the signaling that triggers initiation of acute pancreatitis, but reduces the risk of organ failure and improves the likelihood of survival.
Conclusions and next steps
In summary, this study revealed several significant details about the progression of visceral adipose injury, rapid fat necrosis, worsening inflammation and organ failure that occurs during acute pancreatitis.
"The most immediate impact of these findings on clinical care will involve utilizing the best tools we have to counteract NEFA toxicity," explains Dr. Singh. "While at this time we are largely unequipped to do so, the one agent that helps us is Ringer's lactate. Because the calcium in Ringer's binds the fatty acids to some extent, it provides some benefit. Making Ringer's lactate the primary fluid replacement in patients with acute pancreatitis could help in improving outcomes to a certain extent."
Dr. Singh also notes that because the coronavirus that caused the severe acute respiratory syndrome (SARS) epidemic in 2002 was found in 3 out of 4 of the pancreata of patients who died of SARS on autopsy, there is a high likelihood that coronavirus disease 2019 (COVID-19) also may infect the pancreas asymptomatically. "Both severe acute pancreatitis and COVID-19 are associated with sepsis, multisystem organ failure and lymphopenia. And NEFAs cause direct injury to peripheral blood mononuclear cells, which include lymphocytes," explains Dr. Singh. "Thus, it is very plausible that this pathophysiology may underlie the adverse outcomes and mortality associated with COVID-19. Clinical clues to this may include asymptomatic lipase elevation in patients with COVID-19 who are progressing to organ failure or are in established organ failure versus those who have a mild course. While we are still in the early stages of experience with the COVID-19 virus, any information gleaned from collecting this data or such samples for further analyses of NEFAs could help in prognosticating and improving COVID-19 outcomes, even if it cannot help with treatment of the primary virus. We would welcome communications from our colleagues on this subject via email."
Having identified PNLIP as the primary mediator in NEFA generation and the severity of acute pancreatitis, Dr. Singh and colleagues are hopeful that PNLIP could serve as a pharmacologic target to improve outcomes after disease onset. "Our next phase of research is focused on the rapid development of a lipase inhibitor designed to prevent the fat necrosis and associated bad outcomes," says Dr. Singh.
In addition, these findings demonstrate another diagnosis negatively affected by obesity. "The impact of obesity is observable in chronic diseases such as diabetes, but also in rapidly developing-onset diseases such as pancreatitis," explains Dr. Singh.
For more information
deOliveira C, et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. Journal of Clinical Investigation. In press.
Singh V. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation: Author's take. The Journal of Clinical Investigation. 2020.