Dec. 03, 2016
Diabetes mellitus in the U.S. causes morbidity and mortality and costs in excess of approximately $170 billion a year. Therefore, identifying ways to prevent diabetes is important.
Adrian Vella, M.D., Endocrinology, Diabetes, Metabolism, and Nutrition, at Mayo Clinic in Rochester, Minnesota, says: "The states of impaired fasting glucose and impaired glucose tolerance are associated with a high rate of progression to type 2 diabetes mellitus. However, the risk is heterogeneous. For example, in Olmsted County, Minnesota, 40 percent of people with a fasting glucose ≥ 110 mg/dL progress to overt diabetes within a 10-year period, as opposed to 5 percent of those with a fasting glucose < 95 mg/dL.
"While environmental factors and obesity play a role in progression from prediabetes to diabetes, genetic factors are indubitably important. To date there are approximately 65 common genetic variants reproducibly associated with type 2 diabetes mellitus. However, the greatest risk is associated with variation in the gene TCF7L2.
"The effect size is significant; for example, in the Diabetes Prevention Program, the TT genotype of TCF7L2 at rs7903146 conferred a 2.41-fold increase in risk of type 2 diabetes mellitus compared with the CC genotype. Given the frequency of the diabetes-associated allele — T — of between 30 and 35 percent in most populations and its effect size, this variant makes a substantial contribution to type 2 diabetes mellitus predisposition in a population."
Type 2 diabetes mellitus is characterized by defects in insulin secretion and action, with impaired postprandial suppression of glucagon. Dr. Vella highlights: "It has been reported that diabetes-associated variation in TCF7L2 impairs post-challenge insulin concentrations in most studies. We hypothesized that the diabetes-associated allele in this locus (rs7903146) impairs insulin secretion, and this defect would be exacerbated by acute free fatty acid (FFA)-induced insulin resistance.
β-cell responsivity (Φ) and insulin action (Si)
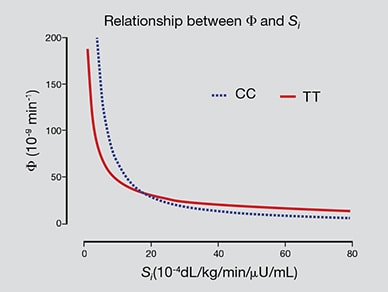
β-cell responsivity (Φ) and insulin action (Si)
The hyperbolic relationship of β-cell responsivity (Φ) and insulin action (Si) in subjects with the TT genotype at rs7903146. Based on Diabetes. 2016;65:371.
"We studied 120 individuals, 60 homozygous for the diabetes-associated allele (TT) at rs7903146 and the remainder homozygous for the protective allele (CC). Using methods independent of insulin concentrations (which reflect both insulin secretion and hepatic extraction of insulin), we have confirmed that subjects who are free of diabetes with the T allele have decreased β-cell responsivity (Φ) for a given degree of insulin action (Si) — the hyperbolic relationship between insulin action and β-cell responsivity is shifted to the left in the TT subjects."
Glucagon in response to a body weight glucose challenge

Glucagon in response to a body weight glucose challenge
Glucagon in response to a 1g/kg body weight glucose challenge with accompanying infusion of intralipid and heparin in subjects with CC (open circles) and TT (solid circles) genotype. Values plotted are means ± SEMs *P < 0.05 for a post hoc unpaired, two-tailed test. Based on Diabetes. 2016;65:371.
Dr. Vella continues: "More importantly, diabetes-associated variation in TCF7L2 impairs postprandial suppression of glucagon secretion — an aspect of islet function that has been ignored in prior genotype-phenotype correlation studies. This is independent of β-cell function and exacerbated by acute insulin resistance — in this case by raising circulating FFA concentrations.
"The contribution of α-cell function (if any) to the progression to diabetes is not known. However, if hyperglucagonemia, alone or in combination with a defect in insulin secretion predisposes to diabetes, this would have significant implications for the prevention of type 2 diabetes mellitus."
For more information
Shah M, et al. TCF7L2 genotype and α-cell function in humans without diabetes. Diabetes. 2016;65:371.