May 22, 2018
A 69-year-old woman presented to the endocrine teaching clinic for evaluation of progressive weakness over 1 1/2 years. She had proximal muscle weakness with inability to stand from a sitting position and she required assistance to ambulate.
More recently, her symptoms progressed to the point where she became wheelchair- or bed-bound most of the time. A stair lift had to be installed in her home. In addition, the patient noted easy bruising with minimal trauma, progressive swelling of her face and lower extremities, and a net loss of weight due to decrease in her muscle mass.
The patient was also recently found to be hypokalemic and was prescribed potassium supplements. She had a two-year history of type 2 diabetes mellitus and long-standing hypertension with recent deterioration necessitating additional pharmacotherapy.
On physical examination the patient appeared chronically ill, sitting in her wheelchair. She had a round face but no plethora. Her skin was very frail with multiple bruises noted in all four extremities but no striation in her abdominal area. There was supraclavicular fullness noted. Her muscular exam revealed severe proximal but only mild distal muscle weakness.
Evaluation confirming adrenocorticotropic hormone-dependent Cushing syndrome
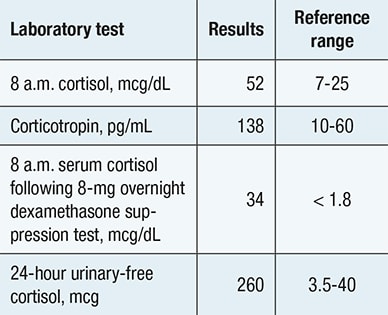
Evaluation confirming adrenocorticotropic hormone-dependent Cushing syndrome
Biochemical evaluation confirming presence of adrenocorticotropic hormone -dependent Cushing syndrome.
Inferior petrosal sinus (IPS) sampling
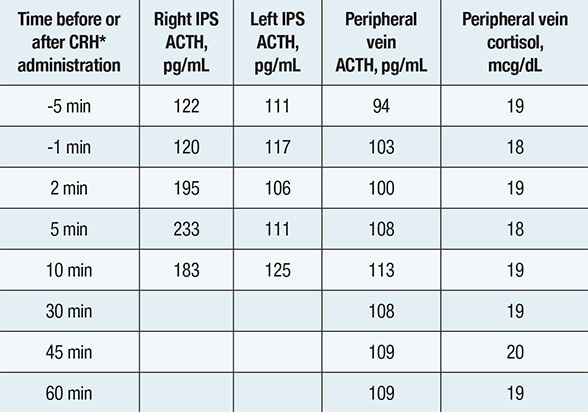
Inferior petrosal sinus (IPS) sampling
Inferior petrosal sinus (IPS) sampling showing comparable corticotropin (ACTH) concentrations in the left IPS, right IPS and peripheral vein — indicating a nonpituitary source of ACTH hypersecretion. *Corticotropin-releasing hormone
Axial CT image showing right lower lobe nodule
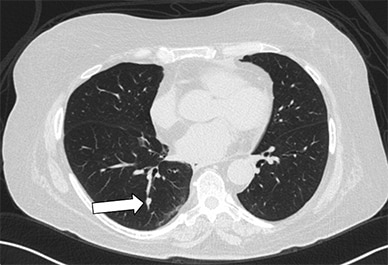
Axial CT image showing right lower lobe nodule
Axial CT image showing a 7-by-5-mm right lower lobe nodule (arrow) that lies immediately adjacent to a vessel and an airway.
CT-guided cryoablation of lung nodule
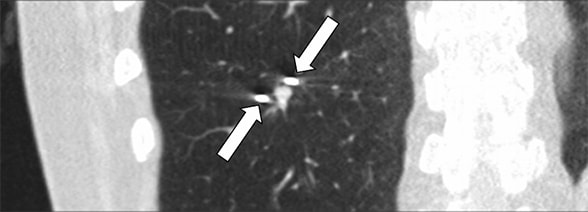
CT-guided cryoablation of lung nodule
CT-guided cryoablation of the 7-by-5-mm lung nodule. Two cryoprobes (arrows) are seen on each side of the nodule.
Biochemical evaluation confirmed corticotropin (ACTH)-dependent Cushing syndrome. Pituitary-directed MRI demonstrated a normal pituitary gland, and inferior petrosal sinus sampling indicated a nonpituitary source of ACTH secretion. In search of an ectopic neuroendocrine tumor, a chest CT scan identified a 7-by-5-mm nodule in the right lower lobe.
Additional findings included hypertrophied bilateral adrenal glands but no other foci of potential disease. Notably, both 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT and 111In-DTPA-pentetreotide scintigraphy failed to demonstrate focal activity in the lung lesion or anywhere else.
Based on a multidisciplinary discussion involving endocrine surgery, thoracic surgery, interventional radiology and informed patient decision-making, it was decided to proceed with a minimally invasive procedure targeting the lung lesion using CT-guided cryoablation. The procedure was performed using three freezes of three to six minutes each, separated by five minutes of thawing, achieving an ablation zone that encompassed the entire tumor without evidence of residual disease.
The patient tolerated the procedure exceedingly well and was discharged from the hospital the following morning. The morning after the procedure, the patient had developed glucocorticoid withdrawal symptoms indicating resolution of the hypercortisolism. The serum cortisol concentration decreased to 6 mcg/dL and the serum ACTH concentration to 13 pg/mL 24 hours after the cryoablation.
The patient went home on a long-term corticosteroid taper. She was also provided with recommendations for intensive physical therapy and Pneumocystis jiroveci pneumonia and venous thromboembolism prophylaxis for the duration of the supraphysiological corticosteroid treatment. Within three months she had lost 12 pounds, improved her muscle function and resumed ambulation. She did not require pharmacotherapy to treat her diabetes. Currently, 16 months after her thermal ablation treatment, the patient continues to improve.
Conclusions
Ectopic tumors account for about 20 percent of all cases of ACTH-dependent Cushing syndrome. The vast majority of these are located in the thorax, with bronchial neuroendocrine tumors being the leading cause (up to 54 percent), as noted by Maria Vittoria Davi, M.D., and colleagues in the European Journal of Endocrinology in 2017 and Jaroslaw Aniszewski, M.D., and others in the World Journal of Surgery in 2001.
Due to their small size, effective preoperative localization can be problematic. The patient reported here was evaluated before the Food and Drug Administration approval of gallium 68 (68-Ga) 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-octreotate (DOTATATE) for PET-CT. Andrea M. Isidori, M.D., Ph.D., and others reviewed conventional and nuclear medicine imaging in ectopic Cushing syndrome in The Journal of Clinical Endocrinology & Metabolism in 2015. A 68-Ga DOTATATE-PET-CT scan may or may not have localized the ACTH-secreting bronchial neuroendocrine tumor in our patient.
In concert with advances in targeted imaging for neuroendocrine tumors, there have been advances in minimally invasive therapy allowing delivery of cryoablation probes to small tumors. Cryoablation induces cancer cell death by activating a number of molecular and cellular processes during the freeze-thaw cycles leading to cancer cell destruction and tissue necrosis, with protection of surrounding normal lung tissue.
Subsequent inflammatory and immune reactions continue for hours to days following the procedure to achieve local tumor control — as reported by Thierry de Baere, M.D., and colleagues in the Journal of Thoracic Oncology in 2015 and Masanori Inoue and others in BioMed Research International in 2014.
For more information
Davi MV, et al. Prognostic factors in ectopic Cushing's syndrome due to neuroendocrine tumors: A multicenter study. European Journal of Endocrinology. 2017;176:453.
Aniszewski JP, et al. Cushing syndrome due to ectopic andrenocorticotropic hormone secretion. World Journal of Surgery. 2001;25:934.
Isidori AM, et al. Conventional and nuclear medicine imaging in ectopic Cushing's syndrome: A systematic review. The Journal of Clinical Endocrinology & Metabolism. 2015;100:3231.
De Baere T, et al. Evaluating cryoablation of metastatic lung tumors in patients — Safety and efficacy: The ECLIPSE trial — Interim analysis at 1 year. Journal of Thoracic Oncology. 2015;10:1468.
Inoue M, et al. Cryoablation of early-stage primary lung cancer. BioMed Research International. 2014;521691.