Feb. 22, 2020
Prostate cancer is the second-leading cause of cancer death in the United States. Men with localized disease often undergo successful definitive treatment. However, men who develop either a recurrence after definitive treatment or metastatic disease often require long-term therapy and can have poor long-term oncologic outcomes.
Androgen deprivation therapy is a stalwart therapy for the initial treatment of metastatic disease. Unfortunately, most men in this situation will go on to develop metastatic castration-resistant prostate cancer (mCRPC) and require secondary systemic therapy. This disease state can be very challenging to treat.
While several relatively new medications exist for patients with mCRPC, current guidelines offer limited direction for optimal treatment sequencing. Some of the common treatments for mCRPC include chemotherapy (docetaxel), androgen synthesis inhibitors (abiraterone) or androgen signal blockers (enzalutamide, apalutamide). Many patients will ultimately receive second line or third line therapies in the course of their mCRPC treatment.
A study published in The Lancet Oncology in 2019 compared two sequences of treatment in mCRPC: abiraterone followed by enzalutamide, or the reverse order of medications. The authors reported a significantly longer time to prostate-specific antigen (PSA) progression when using abiraterone prior to enzalutamide. This work has helped spark significant interest in the sequencing of secondary and tertiary treatments for mCRPC.
Often in clinical practice, providers may initiate either a second generation androgen deprivation therapy (such as abiraterone or enzalutamide) or docetaxel and then switch to the other treatment at the time of evidence of clinical progression. Researchers at Mayo Clinic's campuses in Minnesota and Arizona collaborated to evaluate the optimal sequencing of these commonly used medications.
Oncologist Alan H. Bryce, M.D., at Mayo Clinic in Phoenix/Scottsdale, Arizona, and urologists Eugene D. Kwon, M.D., and R. Jeffrey Karnes, M.D., at Mayo Clinic in Rochester, Minnesota, led a team that investigated the two sequences of treatments:
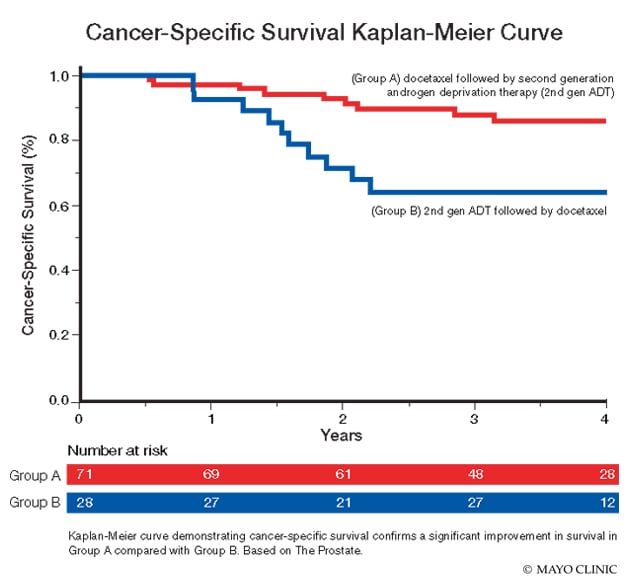
- Group A: Docetaxel before enzalutamide or abiraterone
- Group B: Enzalutamide or abiraterone before docetaxel
The research team performed an institutional database review that found 80 eligible patients in Group A and 32 patients in Group B. Patients in the study had been switched from one form of treatment to the other based on radiographic progression of disease on C-11 PET choline or conventional imaging.
Median follow-up for the cohort was 3.4 years for both Group A and Group B. The Groups were similar with respect to mean age, mean PSA, median Gleason score, pre-treatment number of metastasis and other clinicopathologic variables.
La curva de Kaplan-Meier demuestra la supervivencia específica del cáncer.
La curva de Kaplan-Meier demuestra la supervivencia específica del cáncer.
La curva de Kaplan-Meier que demuestra la supervivencia específica del cáncer confirma una mejora significativa de la supervivencia en el Grupo A en comparación con el Grupo B. Basado en la próstata.
The researchers reported a significant improvement in survival in Group A compared with Group B. The three-year cancer-specific survival rate was significantly higher in Group A compared with Group B (87.4% versus 64.1%; HR 3.6, 95% confidence interval, or CI 1.7-9.5, P = 0.01). The three-year overall survival rate was also significantly higher in Group A compared with Group B (82.4% versus 60.8%; HR 2.29, 95% CI 1.1-4.8, P = 0.03).
These results support the use of docetaxel as the primary first line therapy for mCRPC.
"These results are consistent with the principle of early intensification seen in the castrate-sensitive literature with trials such as CHAARTED and STAMPEDE," says Dr. Karnes. "The results of this study will really impact our practice and provide better outcomes and longer survival for our patients. We hope to continue to refine our treatments for patients with mCRPC in order to provide them with the longest and highest quality of life possible."
The study was published in The Prostate in 2020.
For more information
Khalaf DJ, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase 2, crossover trial. The Lancet Oncology. 2019;20:1730.
Andrews JR, et al. Systemic treatment for metastatic castrate resistant prostate cancer: Does sequence matter? The Prostate. In press.