June 13, 2020
Although immunotherapy is revolutionizing cancer care, its use in glioblastoma has lagged behind the progress seen in other types of cancer. Mayo Clinic is advancing new applications of immunotherapy for glioblastoma, with a focus on more-potent and combination therapies for optimal effectiveness.
"We certainly believe that immunotherapy can be useful for neuro-oncology, as it is now for other indications in solid tumor oncology," says Maciej M. Mrugala, M.D., Ph.D., a neuro-oncologist at Mayo Clinic in Phoenix/Scottsdale, Arizona. "We are beginning to understand some of the mechanisms that make glioblastoma relatively nonimmunogenic, and finding potential ways to activate the cascade of the immune system for self-healing."
Mayo Clinic's enterprisewide efforts range from laboratory exploration to clinical trials in patients with newly diagnosed and recurrent glioblastoma. Preliminary results of separate clinical trials assessing a dendritic cell vaccine and checkpoint inhibitor combination therapy have been promising.
Inyección de la fracción estromal vascular en la cavidad quirúrgica en el momento de la resección del glioblastoma
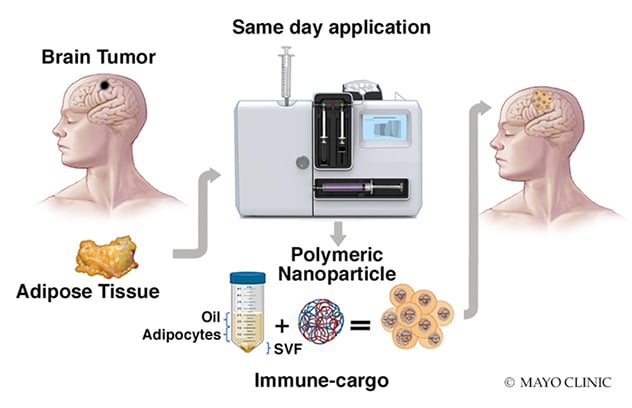
Inyección de la fracción estromal vascular en la cavidad quirúrgica en el momento de la resección del glioblastoma
En un procedimiento desarrollado en Mayo Clinic, se extrae tejido adiposo de un paciente con glioblastoma. En un día, el tejido se procesa en el laboratorio para producir la fracción estromal vascular. La fracción estromal vascular se combina con nanopartículas y se coloca directamente en la cavidad quirúrgica del paciente.
"We're beginning to find that combining immunotherapy with standard glioblastoma therapy provides synergy," says Ian F. Parney, M.D., Ph.D., a neurosurgeon at Mayo Clinic in Rochester, Minnesota. "Some of these findings could be translated into routine clinical practice within the next few years." Other immunotherapeutic strategies under investigation at Mayo include chimeric antigen receptor (CAR)-T cell therapy, injections of stromal vascular fraction (SVF) into the surgical cavity at the time of glioblastoma resection, and the role of extracellular vesicles in brain tumor immunology.
Beyond testing new immunotherapies, Mayo Clinic researchers are seeking explanations for glioblastoma's low immunogenicity. "We must understand how the immune system is being tricked so that the brain cannot fight this cancer," says Alfredo Quinones-Hinojosa, M.D., chair of Neurosurgery at Mayo Clinic in Jacksonville, Florida. "The future is going to involve directly affecting the microenvironment of these cancers."
A more potent vaccine
Glioblastoma's low mutational variance limits the number of antigens that can be targeted, making successful immunotherapy challenging. But Mayo Clinic has developed a dendritic cell vaccine that showed promising results in a preliminary clinical trial in patients with newly diagnosed glioblastoma.
"The average overall survival of the 20 patients in the trial was substantially longer than we would expect for patients receiving standard treatment," Dr. Parney says. "In addition, about 20% of patients in the study survived from four to five years, which is quite unusual in glioblastoma."
The vaccine is based on dendritic cells taken from an individual patient, enhanced in the laboratory and then combined with tumor proteins from a Mayo Clinic library of clinical grade brain tumor cell lines. A larger study is planned in patients with newly diagnosed glioblastoma. Dr. Parney notes that a separate trial of the vaccine in patients with recurrent glioblastoma, already underway, has also had encouraging early results.
Checkpoint inhibitors and CAR-T cell therapy
Immune checkpoint inhibitors, which have had generally disappointing outcomes in glioblastoma, may be more efficacious when combined with standard brain cancer therapies. Early results from a Mayo Clinic phase II clinical trial of pembrolizumab indicate that surgery before administration of that checkpoint inhibitor might boost its effectiveness.
"We don't have complete results yet from the trial, but it's very exciting that we're seeing this effect already," Dr. Parney says. He suggests that the release of tumor proteins during resection might work with pembrolizumab to stimulate the immune system. The clinical trial is also assessing how well pembrolizumab works in conjunction with radiation and chemotherapy.
Like checkpoint inhibitors, CAR-T cell therapy has shown benefit in patients with certain cancers. Mayo Clinic, a pioneer in CAR-T cell therapy for acute lymphoblastic leukemia and non-Hodgkin's lymphoma, is now working to apply that approach to glioblastoma.
"We are hoping to launch a clinical trial that would involve placing CAR-T cells directly into the tumor or spinal fluid, or possibly giving the therapy systemically," Dr. Mrugala says. "There is evidence that CAR-T cells entered into the bloodstream can find their way to spinal fluid and brain tissue and cause very positive responses in patients."
SVF in the surgical cavity
At Mayo Clinic's campus in Florida, researchers are using animal models to investigate immunotherapies placed directly into the surgical cavity at the time of glioblastoma resection. "The most effective window of therapy is while the patient is in the operating room, when the surgical cavity is open," Dr. Quinones-Hinojosa says. "This approach is an opportunity to do something about the residual cancer cells left behind when we debulk the tumor."
One strategy under investigation involves modifying adipose-derived mesenchymal stem cells to secrete anti-tumor proteins. More recently, the researchers have begun studying the effects of SVF placed in the surgical cavity. That work required designing a laboratory methodology to assess the therapeutic mechanisms of SVF on glioblastoma cells.
The SVF used in these studies can be grown directly on the animal models' brains as well as in the laboratory. "Both avenues could unravel the effect the immune cells impose on cancer growth," Dr. Quinones-Hinojosa says. "This has the potential to be an amazing new tool for manipulating the immune system."
Another potential tool is extracellular vesicles. "We have some exciting data showing that extracellular vesicles released by brain tumor cells are important in shutting down the immune system or reducing immunosuppressive changes in monocytes," Dr. Parney says.
Extracellular vesicles might also provide the basis for a liquid biopsy. Mayo Clinic researchers have identified a panel of approximately 45 microRNAs in extracellular vesicles that are dysregulated in glioblastoma and detectable in blood. Unlike MRI, extracellular vesicles can potentially differentiate actual tumor growth from pseudoprogression after treatment.
Dr. Parney notes that pseudoprogression is common during clinical trials that attempt to stimulate immune responses. "A blood test that can distinguish between tumor and inflammation would be very helpful," he says.
Cerebro de un hombre con linfoma de células B antes y después del tratamiento con terapia de células T con CAR
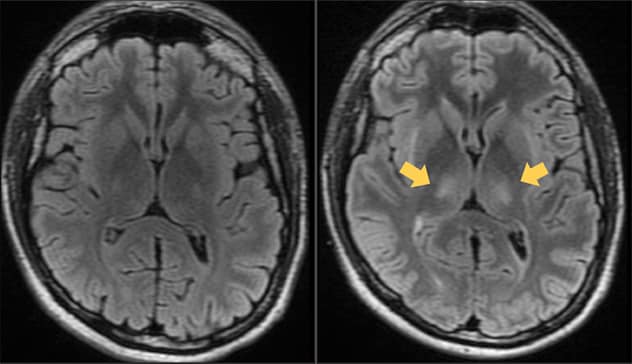
Cerebro de un hombre con linfoma de células B antes y después del tratamiento con terapia de células T con CAR
A la izquierda, la resonancia magnética de recuperación de inversión atenuada por líquido (FLAIR, por sus siglas en inglés) muestra el cerebro de un hombre de 26 años con linfoma de células B antes del tratamiento con terapia de células T con CAR en Mayo Clinic. A la derecha, la resonancia magnética FLAIR siete días después de la terapia de células T con CAR muestra lesiones talámicas bilaterales (flechas) que se desarrollaron en el momento de los síntomas clínicos consistentes con la neurotoxicidad. Los síntomas del paciente se resolvieron en unos días, y las resonancias posteriores fueron normales.
Mayo Clinic's leadership in applying immunotherapy to glioblastoma rests on the enterprise's commitment to translational science. "We have a large team of clinician-scientists — neuro-oncologists, radiation oncologists and immunologists — as well as basic scientists, all working together," Dr. Mrugala says. "In addition, knowing how to care for patients in these clinical trials is critical. Our teams of physicians and support staff understand the side effects of immunotherapies. The safety and comfort of our patients is always paramount."