Dec. 06, 2025
Radioactive iodine (RAI) redifferentiation therapy (RDT) has emerged as a potential salvage strategy using targeted molecular inhibitors to induce sensitivity to otherwise RAI-refractory disease. A Mayo Clinic study that builds on foundational preclinical work and several pilot clinical trials contributes significantly to the field as the largest real-world case series of patients with advanced disease, with a median follow-up of two years. The study, published in Thyroid, provides insights into which patients benefit most and raises important questions about long-term efficacy and safety.
"For decades, RAI therapy has been used for advanced thyroid cancers, but it is often not curative and has limited efficacy in most patients. While newer systemic therapies are effective for RAI-refractory disease, they are also generally noncurative and can be limited by significant adverse effects. The central question of our study was to understand the benefits of RDT in a real-world oncology practice. In other words, who benefits the most and what is the benefit?" says David Toro Tobon, M.D., an endocrinologist at Mayo Clinic and lead author of the study.
The power of the profile: Genotype and histology predict success
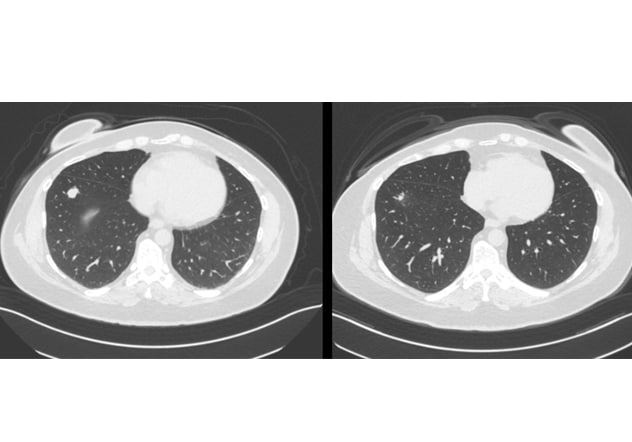 Lung lesion
Lung lesion
The scan on the left shows a pre-redifferentiation right lung lesion. The scan on the right shows the same lesion six months after redifferentiation plus radioactive iodine, demonstrating a significant structural response.
In this retrospective study of 33 patients with progressive, metastatic RAI-refractory differentiated thyroid cancer (RAIR-DTC), successful redifferentiation is strongly linked to a phenotype-genotype. The results show a sharp distinction between subgroups:
- High responders: All 11 patients (100%) with RAS-mutant tumors restored RAI uptake following MEK inhibition. Similarly, all patients with follicular thyroid cancer (FTC) and invasive encapsulated follicular variant papillary thyroid cancer (IEFV-PTC) redifferentiated, as these are commonly RAS mutated tumors.
- Modest responders: In contrast, only 39% (7 of 18) of BRAF-mutant tumors had restored RAI avidity following dabrafenib plus trametinib.
Thus, RDT appears to characterize two groups: a highly responsive follicular/RAS-driven phenotype and a less responsive papillary/BRAF-driven one, making genomic profiling an essential prerequisite for patient selection.
A 2-step benefit: Quantifying the antitumor response
The research uncovered a dual mechanism of benefit: an intrinsic antitumor effect from the targeted inhibitors, followed by an additional response to RAI in patients who redifferentiated. After three weeks of drug therapy, both redifferentiated and nonredifferentiated groups saw a median tumor shrinkage of about 12%. However, the redifferentiated group, after receiving high-dose RAI, achieved a total median shrinkage of 30% at six months. The nonredifferentiated group showed no further improvement.
"Our data suggests a two-pronged benefit," says Mabel Ryder, M.D., an endocrine oncologist at Mayo Clinic and the senior author. "The targeted inhibitors themselves provide an immediate, albeit modest, antitumor effect across the board. But for select patients, restoring iodine avidity allows treatment with high-dose RAI, enabling greater tumor regression that was sustained off targeted therapy."
A paradox of success: Unanswered questions on long-term efficacy and safety
Despite promising initial responses, the study revealed cautionary long-term findings. There were no significant differences between the redifferentiated and nonredifferentiated groups in progression-free survival or time to subsequent systemic therapy.
Moreover, there were more deaths in the RDT group compared with the non-RDT group. In addition, two patients in the RDT group progressed to lethal anaplastic thyroid cancer (ATC) within two years. The nonredifferentiated group experienced zero deaths or transformations. This raises a critical question about tumor heterogeneity and whether RAI — as seen with standard of care and now RDT — may result in selection of aggressive, RAI-resistant tumor subclones and induce genomic changes, leading to eventual anaplastic transformation.
It is important to note, however, that this study did not have a control arm for the follicular-RAS phenotype, which may underscore the benefits of RDT for this cohort. Moreover, this entire cohort represents a group of patients with much more advanced disease and a high risk of mortality.
Conclusion and future directions
This study confirms that RDT can restore RAI avidity and induce significant tumor responses in select patients with RAIR-DTC, particularly those with RAS-mutant, follicular phenotypes. However, whether this therapy improves overall survival in the long term remains a major question. An improved strategy may be to apply RDT with dosimetry earlier in the course of the disease, before referral to oncology, to maximize potential benefits. A pending placebo-controlled study may clarify the best responders for this approach and long-term safety.
For more information
Toro-Tobon D, et al. Clinical outcomes of radioactive iodine redifferentiation therapy in previously iodine refractory differentiated thyroid cancers. Thyroid. 2024;34:70.
Refer a patient to Mayo Clinic.