July 01, 2020
Steven R. Alberts, M.D., is the chair of Medical Oncology and the interim chair of the Mayo Clinic Cancer Center at Mayo Clinic in Rochester, Minnesota.
Editor's note: This interview was originally published in OncLive on June 19, 2020, by Rachel Narozniak, M.A. It has been updated and is shared here with permission from OncLive.
Institutions are slowly but steadily resuming their investigational efforts, examining both the temporary and lasting effects the pandemic has left on their centers.
From restrictions imposed by pharmaceutical sponsors to suspensions and slowed enrollment, the coronavirus disease 2019 (COVID-19) has affected clinical trial operation in oncology centers across the country. Institutions are slowly but steadily resuming their investigational efforts, examining both the temporary and lasting effects the pandemic has left on their centers.
Declining accrual rates, delays in vital inclusion criteria testing, and social distancing protocols limiting the number of in-person office visits that trials often require, are a few examples of the hurdles investigators are currently navigating as operations take steps toward the status quo. In interviews with OncologyLive, investigators from several cancer centers provide insights on how their operations were affected and the strategies that could be sustained in the future.
Inscripción de pacientes en ensayos clínicos en todo el mundo
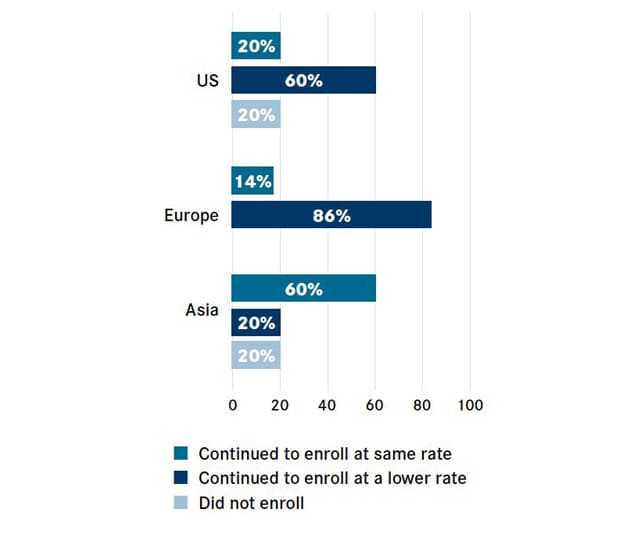
Inscripción de pacientes en ensayos clínicos en todo el mundo
La COVID-19 interrumpe la inscripción de pacientes en los ensayos clínicos en todo el mundo (%). El gráfico se reimprimió con permiso de OncLiv.
New data from the Cancer Research Institute and IQVIA show that just 20% of United States-based centers continued to enroll patients at the usual rate, while 20% halted new patient enrollment altogether. The hardship faced by oncology institutions globally mirrored that of United States oncology institutions: 20% of centers in Asia suspended enrollment, and although the European sites surveyed did not stop enrolling patients, 86% said the enrollments proceeded at a lower rate, according to a recent article published in Nature Reviews Drug Discovery.
Keep lifesaving options on the table
The Mayo Clinic Cancer Center adopted a similar approach to other cancer centers by giving preference to the studies that may hold the most promise, said Steven R. Alberts, M.D., chair of Medical Oncology and deputy director for Clinical Research at the Mayo Clinic Cancer Center, which has sites in Rochester, Minnesota; Jacksonville, Florida; and Phoenix/Scottsdale, Arizona.
"Like any institution, COVID-19 certainly had a big impact on operations, including operations at Mayo's three main campuses in Minnesota, Phoenix and Jacksonville. When COVID-19 hit, it really caused us to greatly slow down our clinical trials," Dr. Alberts said. "We didn't put a complete stop to them, but it (prompted) us to look carefully at which trials were really critical to patients, and in general, our (feeling) was if there were no reasonable standard-of-care options for a patient, we would offer a clinical trial if that trial had some meaningful chance of benefiting a patient."
During this period, Mayo Clinic halted translational data collection and primarily provided phase II and phase III trial offerings, and "very limited" phase I studies. In lieu of suspending studies, Mayo Clinic asked clinicians to internally consult with the clinical research chairs on a new patient enrollment to a given trial and obtain approval from the research chair. This method allowed Mayo Clinic to ensure that only patients who did not have viable therapeutic alternatives were being enrolled on the Cancer Center's clinical trials without wholly shutting down study operations. Unsurprisingly, Mayo Clinic saw a dip in patient accruals: "[At] the lowest point, our enrollment dropped to probably a third of our normal enrollment," Dr. Alberts said.
In what was a welcome change to Mayo Clinic's clinical milieu, the Cancer Center has resumed a more normalized state of study operation and began to open most of its clinical trials the week of May 11, 2020. Already, Mayo Clinic has seen an increase in the number of patients seeking care and clinical trial opportunities, according to Dr. Alberts.
"We expected there to be some increase in patients coming back just for general care, including clinical trials, but our numbers have gone up more quickly than what we thought. At the end of this week, our normal volume of patients is at about 85% of what it had been prior to COVID-19, and if we add in the virtual visits, we're up to about 95%, so the practice has ramped up very quickly, and along with that, we're definitely seeing an increase in the number of patients going on to clinical trials," Dr. Alberts said.
Moving forward, Mayo Clinic will continue to temporarily limit its menu of phase I studies that are visit or procedure intensive to minimize the number of patients who repeatedly come through the institution's doors in this early stage of reopening, Dr. Alberts added.
Forging ahead
Broadly, the clinicians agreed that although COVID-19 hindered some facets of study operation, they also indicated that some of the strategies that were implemented amid the pandemic could not only be sustained in the aftermath but also streamline the facilitation of clinical trials in the future.
"Like a lot of other large institutions, Mayo Clinic is slow to change, and COVID-19 made us change very quickly in a lot of ways," Dr. Alberts said. "The lessons (we) learned will help us design the next generation of trials."
Specifically, the use of telemedicine and remote assessments, when possible, may allow cancer centers to reach a broader network of patients who might not have elected to pursue treatment at a certain institution due to distance, Dr. Alberts observed. In addition to the digital collection of data from study participants in the instances when it was feasible, Mayo Clinic also worked with the National Cancer Institute and pharmaceutical companies to ship drugs to patients within a pre-specified radius. "In the past, nobody would have ever considered sending an oral drug to a patient on a clinical trial through the mail, outside some selected situations," Dr. Alberts said, adding that akin to telehealth services, this practice could possibly endure.
For more information
Narozniak R. Oncology centers examine the aftermath of COVID-19 on clinical trials. OncLive.
Upadhaya S. Impact of COVID-19 on oncology clinical trials. Nature Reviews Drug Discovery. 2020;19:376.