July 31, 2021
As the landscape of oncologic care continues to change and expand, adverse reactions to cancer treatments, particularly involving the lung, are becoming increasingly recognized as an area of growing need for research and therapeutic advancement. Several available studies — published in The Open Respiratory Medicine Journal, Chest, and International Journal of Radiation Oncology, Biology, Physics in 2012, and in PLOS One in 2016 — estimate that lung toxicity occurs in 5% to 20% of all patients receiving systemic antineoplastic therapy or radiation.
"With current treatment options essentially limited to supportive care and variable immunosuppression, identification of predisposing risk factors and ways to mitigate them are of utmost importance," says Ashley M. Egan, M.D., Pulmonary and Critical Care Medicine at Mayo Clinic's campus in Rochester, Minnesota.
عتامة الزجاج المصنفر والتكثف
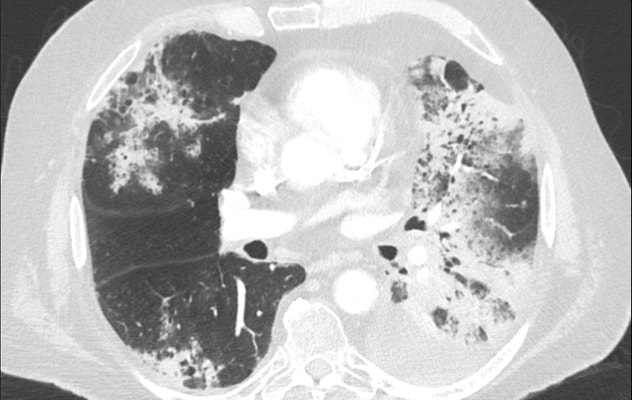
عتامة الزجاج المصنفر والتكثف
عتامة الزجاج المصنفر والتكثف في مريض مصاب بالتهاب رئوي ناتج عن العلاج المناعي.
"Pulmonary toxicity secondary to chemotherapeutic agents has been a long-recognized complication. Incidence varies based on many factors, including the specific medication and dose. Similarly, clinical presentations are diverse, based on the agent administered," notes Dr. Egan. "For example, bleomycin has been shown to cause pneumonitis, diffuse alveolar damage and pulmonary fibrosis, while methotrexate has been associated with hypersensitivity pneumonitis, acute interstitial pneumonia and organizing pneumonia."
Treatment is often discontinuation of the implicated offending agent and supportive care. In cases of severe or progressive disease, immunosuppression, often with glucocorticoids, is pursued, though data supporting such treatment is limited to observational and anecdotal evidence. Despite longstanding use of chemotherapeutic medications, there have not been significant advances in treatment of chemotherapeutic pulmonary toxicity resulting in variable prognoses.
Radiation-induced lung injury has been a recognized adverse effect of radiation therapy for over 100 years. Its incidence is impacted by the following:
- Method of irradiation (stereotactic vs. proton beam)
- Dose of radiation
- Volume of lung treated
- Concurrent systemic chemotherapeutic agents
"Interestingly, patients who seemingly tolerated radiation therapy without complication may develop 'recall pneumonitis' weeks, months or even years after treatment with the introduction of chemotherapy or immunotherapy," says Dr. Egan. "Like chemotherapy-associated lung toxicity, treatment is again generally supportive in nature, as well as is immunosuppression. Improvement in lung function is often observed initially, but beyond 12 to 18 months, further improvement is uncommon."
Immune-related adverse events
عقيدات رئوية متعددة وتضخم العقد اللمفية
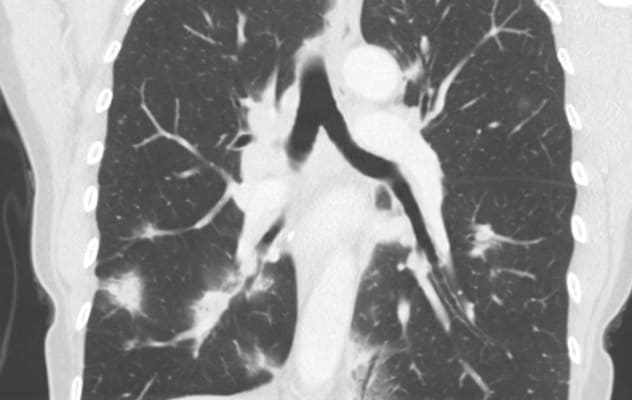
عقيدات رئوية متعددة وتضخم العقد اللمفية
عقيدات رئوية متعددة وتضخم العقد اللمفية لدى مريض يعاني من ردة فعل شبيهة بالساركويد من العلاج المناعي.
Modern immunotherapeutics, specifically checkpoint inhibitors, have dramatically changed the approach to and prognosis of patients with cancer since 2010. As the use of immunotherapy has become more commonplace, recognition of pulmonary side effects (immune-related adverse events) is increasing. Clinical presentations of toxicity range from asymptomatic to respiratory failure. Manifestations are diverse and include pneumonitis, organizing pneumonia and sarcoid-like reaction.
"If identified early, dose reduction or discontinuation often results in resolution," says Dr. Egan. "Unfortunately, in more severe disease, the approach to treatment is again similar in that supportive care and immunosuppression are mainstays. Immunosuppressants beyond glucocorticoids including mycophenolate and tumor necrosis factor-alpha antagonists have been trialed based on observational data, but the use of these medications places the patient at increased risk of life-threatening opportunistic infections."
Pulmonary oncologic toxicity clinic
Mayo Clinic Pulmonary and Critical Care Medicine is establishing a pulmonary oncologic toxicity clinic to care for patients with lung toxicity from chemotherapy, radiation and immunotherapy. A multidisciplinary team composed of experts in Pulmonary and Critical Care Medicine, Medical Oncology, Radiation Oncology, Radiology, and Laboratory Medicine and Pathology will collaborate to identify modifiable risk factors for toxicity; detect adverse effects early to mitigate severity of disease; and align management with evidence-based treatments to improve outcomes.
Interested patients will have the opportunity to enroll in clinical studies that aim to advance understanding and treatment. Early anticipated studies include a prospective observational study assessing patient outcomes with implementation of a treatment algorithm; remote patient monitoring with home pulse oximetry; and creation of a biorepository.
Dr. Egan concludes: "As the oncologic care of patients with advanced malignancy continues to change and expand, prognoses, from an oncological standpoint, continue to markedly improve. Unfortunately, for those with treatment-associated lung toxicity, outcomes have not kept pace due to limited research and existing evidence. Mayo Clinic Pulmonary and Critical Care Medicine is committed to advancing research in chemotherapy, radiation and immunotherapy pulmonary adverse effects to improve overall survival in patients with pulmonary cancers."
For more information
Schwaiblmair M, et al. Drug induced interstitial lung disease. The Open Respiratory Medicine Journal. 2012;6:63.
Dhokarh R, et al. Drug-associated acute lung injury: A population-based cohort study. Chest. 2012;142:845.
Barriger RB, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. International Journal of Radiation Oncology, Biology, Physics. 2012;82:457.
Fujimoto D, et al. Characteristics and prognostic impact of pneumonitis during systemic anti-cancer therapy in patients with advanced non-small-cell lung cancer. PLOS One. 2016;11:e0168465.