Feb. 16, 2021
Infection complicates up to 3% of major spinal surgeries and is associated with significant morbidity and cost. Those issues stem in part from challenges surrounding the diagnosis of infections after spinal implant surgery. Mayo Clinic has found ways not only to dramatically reduce the rate of surgical site infections after spinal procedures but also to better diagnose infections when they occur.
"These situations are often frustrating for patients and physicians," says Paul M. Huddleston III, M.D., an orthopedic surgeon at Mayo Clinic in Rochester, Minnesota. "The X-rays of patients with spinal implant infections may look normal. But the patients have pain around the implant area. The physicians know the patients aren't doing well, but by the standard measures there appears to be nothing wrong."
Mayo Clinic has a long history of research in orthopedic surgical site infections. "Recently we have worked to broaden the knowledge base from lessons learned with knee replacements and other artificial joints to the area of spine instrumentation," Dr. Huddleston says. "Infections associated with spinal implants can be quite painful. In some instances, they can cause paralysis or be lethal."
انخفاض العدوى في مرافق الجراحة
انخفاض العدوى في مرافق الجراحة
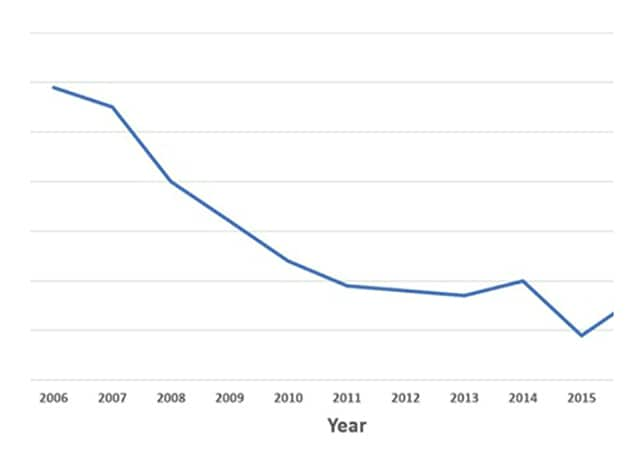
خفضت مايو كلينك من معدل العدوى في مرافق الجراحة بعد جراحة العمود الفقري بمقدار الثلثين من عام 2006 إلى عام 2016.
In the decade from 2006 to 2016, Mayo Clinic reduced the rate of surgical site infections after spinal surgery by two-thirds. That decline — described in the February 2019 edition of The Spine Journal — was achieved in the context of a large, quaternary center engaged in a disproportionate amount of complex spinal surgery in patients with high morbidity.
Mayo Clinic's bundled approach consisted of five simple and cost-effective surgical protocols:
- Application of intrawound vancomycin powder
- Wound irrigation with dilute Betadine solution
- Preoperative chlorhexidine gluconate scrubs
- Preoperative screening with nasal swabbing and decolonization of S. aureus
- Perioperative antibiotic administration
"We can't say which of these individual interventions is most effective," Dr. Huddleston says. "But we clearly showed there's no obvious harm, and an enormous decrease in infection rate, from doing them all in a bundle."
Further research is needed to define the optimal use of prophylactic antibiotics in spinal wounds. "As orthopedic surgeons, we certainly don't want to drive the unwanted development of superbugs. But we are also sensitive to the need to prevent implant-associated infections," Dr. Huddleston says.
Finding elusive bacteria
Many of the organisms commonly implicated in implant infections form biofilms on an implant's surface — making it difficult to obtain a culture from tissue samples alone. A technique developed at Mayo Clinic of vortexing and sonication of implants has demonstrated superior sensitivity and specificity compared with tissue sampling, in many types of orthopedic implants. However, vortexing and sonication has not been widely applied to spinal implants.
Mayo Clinic recently developed a system for testing sonication fluid obtained from spinal implants. "This methodology allows us to blast the biofilm off the spinal implants and culture the bacteria that hide in there," Dr. Huddleston says. "Many patients who do not have access to these types of diagnostics are at risk of being told after tissue sampling that they don't have an infection, when they may in fact have an infection hiding in the biofilm on the implant itself."
In a study published in Spine in 2020, Mayo Clinic researchers found that their method of spinal implant sonication is sensitive and specific for the diagnosis of spinal implant infections. Lower thresholds for defining sonicate fluid culture positively allow for increased sensitivity with a minimal decrease in specificity, enhancing the clinical utility of implant sonication. That study is a part of Mayo Clinic's ongoing research into retained orthopedic implants.
As a major clinical and research center, Mayo utilizes the expertise of specialists in infectious diseases and internal medicine as well as orthopedic surgery. "It's extremely rewarding when we can, in a collaborative fashion, produce a positive outcome for patients that they might not have elsewhere," Dr. Huddleston says. "Mayo Clinic is uniquely positioned to help these patients with very challenging conditions."
For more information
Tomov M, et al. An empiric analysis of 5 counter measures against surgical site infections following spine surgery — A pragmatic approach and review of the literature. The Spine Journal. 2019;19:267.
Carlson BC, et al. Implant sonication versus tissue culture for the diagnosis of spinal implant infection. Spine. 2020;45:E525.