Jan. 11, 2019
Mayo Clinic has the breadth of expertise to quickly assemble a pediatric care team for children with brain tumors. This multidisciplinary approach typically includes pediatric specialists in neurology, hematology-oncology, radiation oncology and neurosurgery.
"We often see these patients within a day of their referral to Mayo Clinic," says David J. Daniels, M.D., Ph.D., a pediatric neurosurgeon at Mayo Clinic in Rochester, Minnesota. "We do the necessary scans and meet as a group to come up with the optimal treatment plan — we don't make decisions individually. Our integrated team is able to mobilize rapidly to provide that expertise."
حشو السرج الموحَّد المعالج للآفة والفراغات فوق الجافية
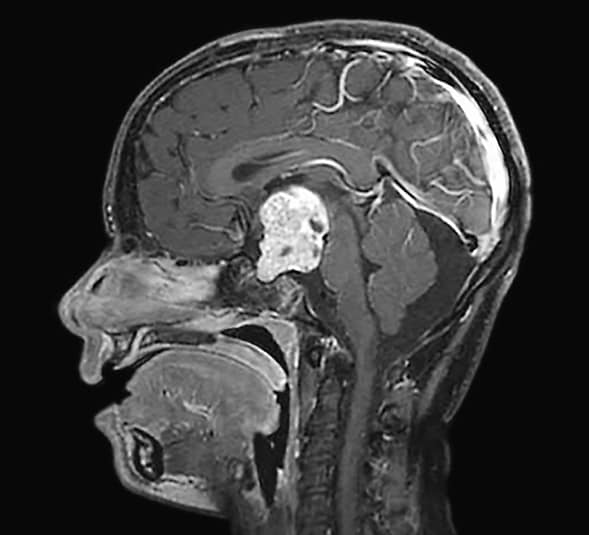
حشو السرج الموحَّد المعالج للآفة والفراغات فوق الجافية
تبين الصورة السهمية للتصوير بالرنين المغناطيسي حشو السرج الموحَّد المعالج للآفة والفراغات فوق الجافية. تم تنفيذ الاستئصال الإجمالي الكلي باستخدام نهج بين النصفين الدماغيين عبر الغلاف المشيمي. وقد قدم تقرير علم الأمراض تشخيص الإصابة بورم قحفي بلعومي خبيث.
As a high-volume center with deep neurological and neurosurgical expertise, Mayo Clinic has experience with the most challenging benign and cancerous brain tumors in children. Dr. Daniels and his team treat patients with tumors located in difficult areas of the brain, including the third and fourth ventricle, skull base and eloquent areas such as speech and motor. Awake craniotomies are occasionally performed in carefully selected pediatric patients to resect lesions near speech centers. An awake craniotomy allows for safe maximal tumor resection while preserving functional tissue.
"The patient we would consider for an awake procedure is older — 14 to 18 years old — and we are very selective in terms of the patient's anxiety with an awake procedure," Dr. Daniels says. "Mayo has a long history of performing awake craniotomies in adults, so we have anesthesiologists and other specialists who can support our work in these selected pediatric patients."
For all pediatric brain tumor surgeries, Mayo Clinic employs state-of-the-art imaging. Functional MRI is used to map speech, motor and sensory pathways before surgery. Advanced intraoperative monitoring, including sensory cortex neuromonitoring and intraoperative MRI, also is routinely used.
"Intraoperative MRI allows us to be very specific in our tumor resection," Dr. Daniels says. "If any residual tumor is seen on intraoperative MRI, we can resect it right away rather than waiting to get an MRI and going back into surgery the next day."
Developing novel therapies for H3K27M tumors
At Mayo Clinic, every pediatric brain tumor with sufficient tissue available is saved for research purposes, with some of the tissue going to the Experimental Drug and Therapeutics for Pediatric Brain Tumor Laboratory, led by Dr. Daniels. The tumors are grown in cell culture, transferred to mouse models and treated with experimental drugs. In an effort to identify potential for rapid clinical translation, the lab has screened thousands of promising drugs in numerous cancer cell lines and uncovered important signaling pathways in these tumors.
About 60 cell lines have been developed, including H3K27M-mutant diffuse midline glioma tumors. Patients with H3K27M-mutant gliomas, formerly classified as diffuse intrinsic pontine gliomas, have a life expectancy of nine to 12 months after diagnosis.
A key focus of the lab's investigation of possible treatments for this devastating disease is signal transducer and activator of transcription 3 (STAT3) proteins. "This transcription factor appears to be very integral for the H3K27M-mutant tumor to grow and survive," Dr. Daniels says. "When we treat lab mice with STAT3 inhibitors or knock this pathway out genetically, we're able to decrease the tumor size and extend life expectancy in these models."
يقلل المثبِّط STAT3 نمو الورم H3K27M
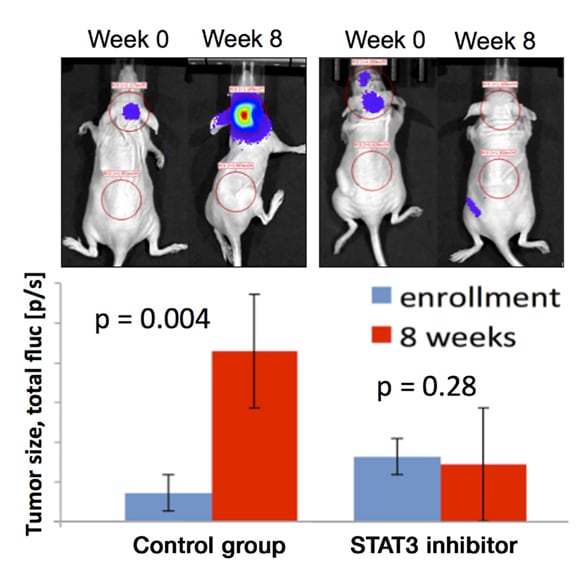
يقلل المثبِّط STAT3 نمو الورم H3K27M
يقلل المثبِّط STAT3 نمو الورم H3K27M في الطعم الأجنبي سوي الموضع المشتق من المريض. لقد تمت زراعة خط الورم H3K27M المشتق من المريض، المنشأ في Mayo Clinic، في الجرذان وتمت معالجته بالمثبِّط STAT3 بإعطائه عن طريق الفم مقابل المراقبة.
Dr. Daniels is proposing a phase 1 clinical trial of a STAT3 inhibitor that has worked effectively in the lab's mouse models. The lab has also synthesized and patented other novel drugs and found them to be potent inhibitors of the STAT3 pathway.
In addition to the STAT3 pathway, the lab is investigating ways to reverse the effects of this histone mutation on the epigenome. That work led the researchers to discover another class of compounds, the Aurora kinase inhibitors, as potential therapeutic targets. Experimental therapies focused on that pathway have succeeded in reducing the size of tumors grown in mouse flanks; experiments on cranial tumors in mice are underway. The lab's findings support the hypothesis that Aurora kinases are critical for epigenetic reprogramming in H3K27M tumor cells and represent a targeted approach for treating tumors with this mutation.
"As with the STAT3 pathway, we have found not just a therapy that seems to work but also a mechanism that explains why certain therapies might work against the H3K27M mutation," Dr. Daniels says. He hopes to propose a clinical trial of a drug targeting Aurora kinases in the next couple of years.
In conjunction with Mayo Clinic's Center for Immunology and Immune Therapies, the Experimental Drug and Therapeutics for Pediatric Brain Tumor Laboratory is also exploring oncolytic viruses and chimeric antigen receptor (CAR)-T cell therapy for H3K27M-mutant tumors.
"By understanding how the H3K27M histone mutation contributes to therapeutic vulnerabilities in tumor cells, we hope to develop new treatment paradigms for children with this devastating disease," Dr. Daniels says.
For more information
Mayo Clinic Center for Immunology and Immune Therapies
Mayo Clinic Experimental Drug and Therapeutics for Pediatric Brain Tumor Laboratory