Aug. 20, 2021
معلومات بالغة الأهمية حول الورم
معلومات بالغة الأهمية حول الورم
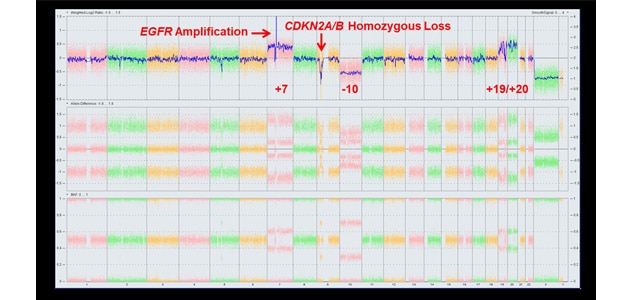
تُظهر نتائج لوحة اختبار ورم الدماغ الوراثي نمطًا نموذجيًا للمصفوفات الصبغية الدقيقة للورم الأرومي الدبقي من النوع الشرس لنازعة هيدروجين الإيزوسيترات لطفرات إنزيم تيلوميراز العكسي.
Mayo Clinic has developed genetic brain tumor test panels that can provide critical information about individual tumors, to better direct patient care. Components of the tests have been included in new brain tumor testing guidelines from national and international cancer-related associations, including the College of American Pathologists.
"In the clinical trial of the panels, our genetic testing provided diagnostic and prognostic information that would not have been found otherwise for about 60% of trial participants," says Robert B. Jenkins, M.D., Ph.D., a medical geneticist and pathologist at the Mayo Clinic Cancer Center in Rochester, Minnesota.
The genetic variability of glioma has made the identification of biomarkers a key component in developing a care plan. Mayo Clinic's panels, available through Mayo Clinic Laboratories, use targeted next-generation sequencing to evaluate mutations and rearrangements in 187 genes, including 104 fusions and 29 transcript variants.
The springboard for developing the tests was a groundbreaking Mayo Clinic study published in the June 25, 2015, issue of the New England Journal of Medicine that showed gliomas could be classified into five main groups based on three tumor biomarkers. At the time, Mayo Clinic used a next-generation sequencing test that examined 50 genes.
"We had no really broad panel to test all mutations and potential fusions in brain tumors," Dr. Jenkins says.
With funding from the Centers for Medicare & Medicaid Services' Transforming Clinical Practice Initiative, Dr. Jenkins led a team of Mayo Clinic medical geneticists, neuropathologists, neuro-oncologists, neurosurgeons and radiation oncologists who built the testing panels. The tests were launched in 2018.
Known as NONCP and CMAPT, the panels include genes that were believed at that time to have clinical significance but lacked official recognition. "We knew that new genetic markers were on the horizon, so we overbuilt NONCP in case those markers became relevant in the future," Dr. Jenkins says.
One example is the cyclin-dependent kinase inhibitor 2A (CDKN2A) gene homozygous deletion, which is identified on Mayo Clinic's test panels.
"In the new version of World Health Organization criteria, CDKN2A loss will bump up a diagnosis from low-grade tumor to glioblastoma. That has profound implications for how we think about the disease and how we should treat it," says Terence (Terry) C. Burns, M.D., Ph.D., a neurosurgeon in the Brain Tumor Program at Mayo Clinic's campus in Minnesota. "Understanding the mutations that are present can also help us identify candidate biomarkers to figure out what a tumor is doing over time."
With the rapid pace of discovery of genes with clinical significance, Mayo Clinic researchers are working to update the panels. "We constantly think about how we can improve our service to provide the best information for doctors who care for patients with brain tumors," Dr. Jenkins says. "Translating research into care for patients is what the Mayo Neuro-Oncology Program does every day."
For more information
Mayo Clinic Laboratories. Mayo Clinic.
Eckel-Passow JE, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. New England Journal of Medicine. 2015;372:26.