Feb. 16, 2019
Gastroesophageal reflux disease (GERD) is a common reason for American adults to see a gastroenterologist and the leading indication for upper endoscopy. In addition to its impact on quality of life, chronic GERD is a risk factor for numerous adverse events, such as esophageal stricture formation, Barrett's metaplasia and esophageal adenocarcinoma, thus necessitating adequate diagnosis and treatment of this common entity.
قصور وظيفة الصمام المعدي المريئي
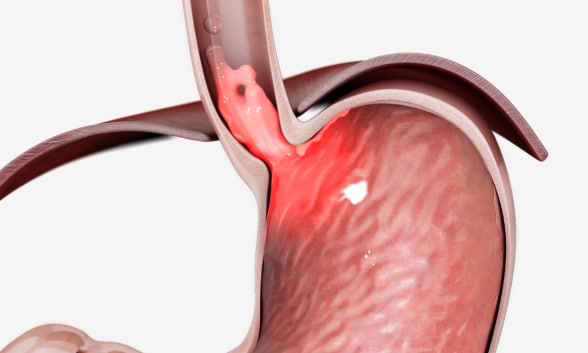
قصور وظيفة الصمام المعدي المريئي
يواجه بعض الأفراد الاستجابة الجزئية أو الأقل من النموذجية لمثبطات مضخة البروتون بسبب قصور وظيفة الصمام المعدي المريئي. مُعادة الطبع وفق إذن. ©EndoGastric Solutions, Inc.
Until recently, proton pump inhibitors (PPIs) were deemed the treatment of choice for the majority of individuals with early disease and mild to moderate chronic symptoms, with plenty of study results demonstrating their efficacy. However, researchers estimate that about a quarter of individuals experience a partial or suboptimal response to PPIs for a variety of reasons, including noncompliance and failure of the drugs to address an underlying incompetent gastroesophageal valve function, which is an anatomical abnormality not corrected by this class of medications. Additionally, although the absolute risk is low, multiple studies have shown an association between long-term PPI use and potential adverse health outcomes.
Surgical interventions, including Nissen fundoplication and the more commonly performed laparoscopic Nissen fundoplication, are available for those individuals who require anatomic correction. This can include individuals who are intolerant to PPIs, those who are concerned about long-term medication use, or those who have advanced anatomical abnormalities of the gastroesophageal junction (such as a large hiatal hernia) preventing appropriate valve functioning. Both of these procedures, however, are associated with complications inherent in surgical intervention and can have chronic side effects such as bloating and dysphagia. And some patients will have recidivism of symptoms requiring re-initiation of medical therapy.
These concerns have fueled interest in the search for a noninvasive endoscopic therapeutic alternative that's both safe and effective in bridging the current gap in GERD management for appropriately selected patients. Endoscopic methods to control reflux in the United States include the Stretta procedure, Medigus Ultrasonic Surgical Endostapler (MUSE) and transoral incisionless fundoplication (TIF).
The American Society for Gastrointestinal Endoscopy guidelines published in 2015 note that endoscopic anti-reflux therapy may be considered for select patients with GERD. In this Q&A, Barham K. Abu Dayyeh, M.D., M.P.H., a gastroenterologist specializing in advanced endoscopic procedures at Mayo Clinic's campus in Rochester, Minnesota, answers key questions about TIF and provides an overview of recent research findings evaluating the efficacy of this approach.
What is the goal of TIF and how is it performed?
استعادة تكامل الصمام المعدي المريئي بتثنية القاع دون جراحة عبر الفم
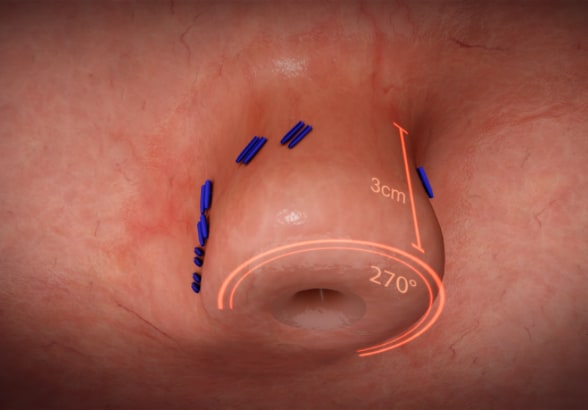
استعادة تكامل الصمام المعدي المريئي بتثنية القاع دون جراحة عبر الفم
يتم إجراء تثنية القاع دون جراحة عبر الفم (TIF) لاستعادة تكامل الصمام المعدي المريئي. مُعادة الطبع وفق إذن. ©EndoGastric Solutions, Inc.
جهاز EsophyX Z+
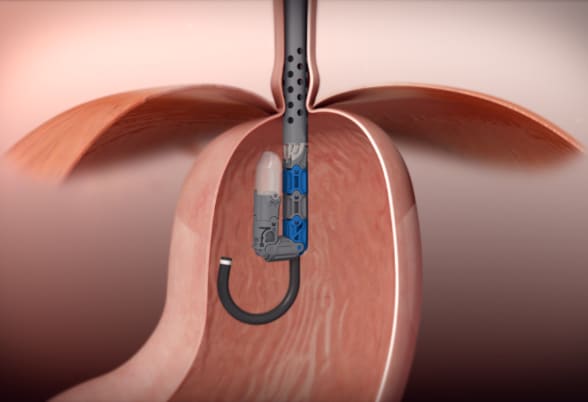
جهاز EsophyX Z+
يتم إجراء تثنية القاع دون جراحة عبر الفم من خلاله دون فتح شقوق جراحية باستخدام جهاز EsophyX Z+. مُعادة الطبع وفق إذن. ©EndoGastric Solutions, Inc.
The goal of TIF is to restore the integrity of the gastroesophageal valve by creating a 270-degree esophagogastric wrap around the distal esophagus, anchored by multiple polypropylene fasteners, which have similar strength to 3.0 sutures. The procedure is performed through the mouth with no surgical incisions using the EsophyX Z+ device.
Which patients can benefit from TIF?
Individuals who can benefit from the TIF procedure include:
- Patients with typical GERD symptoms and suboptimal control of their symptoms on PPIs who do not have a large hiatal hernia or esophageal motility problems
- Patients with GERD symptoms and mild to moderate anatomic abnormalities of the gastroesophageal junction valve function that require correction
- Patients preferring an alternative management strategy for their GERD that does not involve long-term medication use
At Mayo Clinic, we have been offering the TIF procedure for a few months. Multiple patients have undergone the procedure with no complications and achieved excellent control of their GERD symptoms in the short term.
What do recent research results show about the safety and efficacy of TIF?
Results from the Randomized EsophyX vs. Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial published in Gastroenterology in 2015 demonstrated that TIF was an effective treatment for patients with GERD symptoms, particularly in those with persistent regurgitation despite PPI therapy, based on an evaluation six months after the procedure. The trial randomized 87 patients to TIF with a placebo versus 42 patients to a sham procedure and a PPI. A total of 67 percent of patients in the TIF arm no longer had regurgitation compared with 45 percent in the sham arm.
A study published in The American Journal of Gastroenterology in 2015 followed patients for 12 months, as they were randomized to TIF versus PPI. The primary outcome of the study was GERD-related quality of life, which improved significantly with TIF compared with baseline and PPI use only. The authors concluded that although TIF improved GERD-related quality of life and yielded short-term improvements in the anti-reflux barrier in some individuals, no long-term objective reflux control was achieved.
A double-blind, sham-controlled study of patients with GERD who were chronic PPI users randomized 88 patients equally, and 60 percent of patients remained free of symptoms at six months after intervention. Based on these results, published in Alimentary Pharmacology and Therapeutics in 2015, the authors concluded that TIF is effective in people with chronic PPI-dependent GERD when followed for six months.
Karim S. Trad, M.D., and co-authors shared results from two trials worth noting. In an article published in BMC Gastroenterology in 2014, they shared the results of a randomized crossover study involving 40 patients receiving TIF and 23 receiving high-dose PPIs who were followed for six months before crossing over. Six months after TIF, the majority of crossover patients (71 percent) were off PPIs. At the end of the study (at 12 months), only 18 percent of the index TIF group patients were using PPIs.
In 2018, the five-year TEMPO trial results were published in Surgical Innovation by the same authors, offering the longest duration of follow-up to TIF shared thus far. In this randomized controlled crossover study, 44 participants completed the study at five years. Regurgitation resolved in 86 percent of study participants, and atypical symptoms improved in 80 percent of study participants. The authors noted that no serious adverse events were reported during the duration of the study. Only three participants underwent a repeat procedure by the end of the five years. Furthermore, at five years, 66 percent of participants had discontinued their daily PPI intake.
Given these findings, what long-term role do you foresee for TIF in the treatment of GERD?
The wheel of innovation in endoscopy has yielded several revolutionary changes in the minimally invasive management of different GI disorders, and GERD is one of these targets. The TIF procedure offers appropriately selected patients with bothersome symptoms the opportunity to improve their quality of life without resorting to long-term medication use or major surgical interventions. When adequately implemented in the confines of comprehensive multidisciplinary programs, interventions such as TIF will bridge the current GERD management gap and offer an effective solution to many patients suffering with this chronic disorder.
For more information
Hunter JG, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology. 2015;148:324.
Witteman BPL, et al. Randomized controlled trial of transoral incisionless fundoplication vs. proton pump inhibitors for treatment of gastroesophageal reflux disease. American Journal of Gastroenterology. 2015;110:531.
Håkansson B, et al. Randomised clinical trial: Transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Alimentary Pharmacology and Therapeutics. 2015;42:1261.
Trad KS, et al. Efficacy of transoral fundoplication for treatment of chronic gastroesophageal reflux disease incompletely controlled with high-dose proton-pump inhibitors therapy: A randomized, multicenter, open label, crossover study. Gastroenterology. 2014;14:174.
Trad KS, et al. The TEMPO trial at 5 years: Transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surgical Innovation. 2018;25:149.