April 13, 2021
A midterm analysis of a multisite phase 1 study of cevostamab for relapsed or refractory multiple myeloma (RRMM) has not only shown that dose escalation with cevostamab is feasible but also that this agent is well tolerated and demonstrates promising activity. The analysis results were presented at the 2020 American Society of Hematology (ASH) virtual meeting.
"At the ASH meeting, everyone agreed that the bispecific antibody presentations in multiple myeloma — of which this was one of two — were deemed the most striking," says Rafael Fonseca, M.D., a hematologist at Mayo Clinic in Arizona and the interim executive director of Mayo Clinic Cancer Center. "In this study thus far, over 50% of patients had responses."
The presentation at ASH was the first-time report on this antibody.
The report presented data for 51 participants. Study investigators found deep responses in those whose disease responded to cevostamab therapy. Some participants experienced complete response, and some even reached minimal residual disease (MRD) at a sensitivity of 10-5, the highest possible response level. As dosage escalated to a 3.6/90 mg to 3.6/132 mg level, response also increased. Participants treated at these dose levels showed an overall response rate of 61%, with two complete responses and five out of 18 participants demonstrating very good partial response, as defined by the International Myeloma Working Group.
ارتباط الجسم المضاد Cevostamab
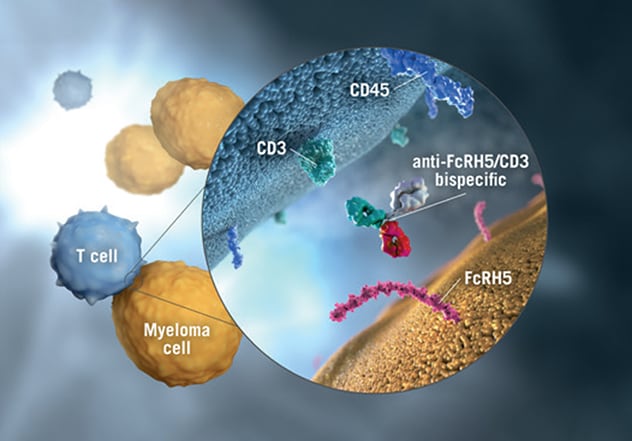
ارتباط الجسم المضاد Cevostamab
يرتبط الجسم المضاد Cevostamab بكل من الجزء القابل للتبلور الشبيه بالمستقبل 5 (FcRH5) الموجود على سطح خلايا الورم النقوي وعنقود التمايز (CD3)، وهو مكون في مركب مستقبل الخلايا التائية (TCR)، الموجود على سطح الخلايا التائية. أُعيدت طباعة الصورة بعد الحصول على إذن من شركة هوفمان-لا روش.
Previous animal studies of this bispecific antibody had demonstrated activity, and testing against B cells had proved successful, says Dr. Fonseca. A bispecific antibody such as cevostamab is designed for complicated, challenging disease and demonstrates tandem binding. It targets CD3 on T cells and the most membrane-proximal domain of FcRH5, a surface marker on myeloma cells. In contrast, in another study published by Dr. Stewart and colleagues in a February 2019 issue of Blood Cancer Journal, investigators studied a naked antibody, the more commonly used type of monoclonal antibody, which showed limited activity in patients with RRMM.
As Dr. Fonseca and colleagues evaluated the safety of cevostamab, they found nearly all study participants experienced at least one adverse event, but most were not serious. The most common side effect was cytokine release syndrome (CRS). Though the investigators took this side effect seriously and continued to monitor affected patients, it did not prevent continued doses and did not have any major consequence — nothing out of the ordinary, as Dr. Fonseca describes it. He indicates that some level of this side effect is actually desirable.
"We want to see CRS to some degree because CRS within reason tells you the drug is acting," says Dr. Fonseca.
The challenge before the investigators
All participants in this study had been through multiple prior lines of therapy — a median of six — relapsing numerous times, and they did not have other therapeutic options.
Dr. Fonseca explains that options such as chimeric antigen receptor (CAR)-T cell therapy are available to such patients. But such an option involves removing T cells from the patient, sending the T cells to a facility for preparation for three weeks, and then infusing the cells back into the patient. The process can be complicated and time-consuming, not to mention expensive.
"Our purpose is to find an off-the-shelf therapy — something we can have in the pharmacy," he says. "We'd like an agent that is quicker to the patient, less bureaucratic, less expensive and simpler."
In addition to these characteristics, in a disease like multiple myeloma for which there is no cure and most patients relapse and ultimately die of their disease, patients simply need more options. Each relapse means that patients not only need more therapy but also almost always need a new therapy to which they haven't been exposed, as the disease often becomes refractory to previous treatments.
Current trial status and patient referral
As the ASH presentation represented a midterm analysis, the phase 1 trial is ongoing, now with expansion arms, and still enrolling patients.
For hematologists treating patients with RRMM, Dr. Fonseca not only recommends referring for the cevostamab phase 1 trial but also for evaluation for any one of many multiple myeloma trials Mayo Clinic is leading or for which it is a participating site.
"As of now, the cevostamab trial is just one of many examples of how Mayo Clinic is engaging in this type of research in multiple myeloma," says Dr. Fonseca.
For more information
Cohen AD, et al. Initial clinical activity and safety of BFCR4350A, a FcRH5/CD3 T-cell-engaging bispecific antibody, in relapsed/refractory multiple myeloma. Presentation at: 62nd American Society of Hematology (ASH) Annual Meeting and Exposition; 2020; livestream.
International Myeloma Working Group (IMWG) uniform response criteria for multiple myeloma. International Myeloma Foundation.
Stewart AK, et al. Phase I study of the anti-FcRH5 antibody-drug conjugate DFRF4539A in relapsed or refractory multiple myeloma. Blood Cancer Journal. 2019;9:17.