Oct. 10, 2025
"Osteoarthritis is one of the most prevalent diseases on the planet and one of the least treatable. It is the leading cause of disability among the elderly and a source of considerable economic loss," says researcher Christopher H. Evans, Ph.D. Those simple but devastating facts have fueled decades of research conducted by Dr. Evans and the research team he leads at Mayo Clinic's Musculoskeletal Gene Therapy Research Laboratory in Rochester, Minnesota.
While focusing on the role of inflammation in the pathophysiology of osteoarthritis (OA), Dr. Evans and colleagues determined that interleukin-1 (IL-1) plays a key role as an intra-articular mediator of inflammation, pain and the loss of cartilage. This finding meant that the molecule's natural inhibitor, the IL-1 receptor antagonist (IL-1Ra), could serve as a promising therapeutic.
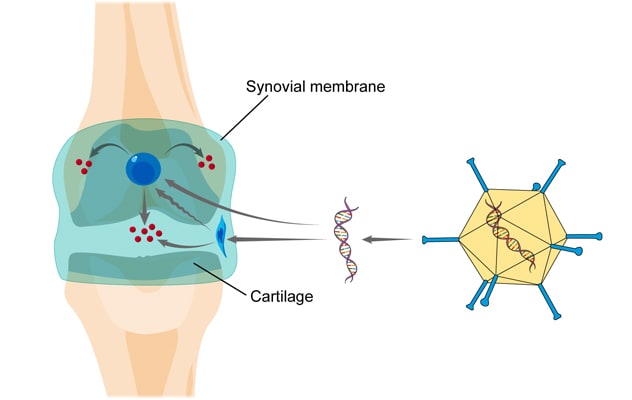 Intra-articular gene therapy
Intra-articular gene therapy
The image shows the basic concept behind intra-articular gene therapy for joint diseases such as arthritis. A vector containing DNA that encodes the therapeutic gene product is injected into the affected joint where it delivers its genetic payload to synovial lining cells and articular chondrocytes. These cells then synthesize the therapeutic gene product locally in a sustained fashion.
However, because most proteins have limited half-lives in vivo, finding a way to deliver sustained, therapeutic concentrations of IL-1Ra directly to joints was an additional challenge to overcome. According to Dr. Evans and colleagues, gene therapy offers an elegant solution to this problem. They developed and are studying a gene transfer vector — the self-complementary recombinant adeno-associated virus sc-rAAV2.5IL-1Ra — that delivers the IL-1Ra gene to joints, stimulating the recipient's articular cells to synthesize antiarthritic gene products endogenously.
"The gene therapy approach is novel, both as a strategy for treating osteoarthritis and as an application of gene therapy. It has previously been used to address only genetic diseases and cancer," explains Dr. Evans.
In their most recent phase of research, Dr. Evans and colleagues conducted a single-center, first-in-human phase 1 clinical trial to test the safety of this approach in people with midstage OA. The results of this study were published in Science Translational Medicine and highlighted in Molecular Therapy in 2025.
Study methods
The phase 1 trial involved nine study participants with radiographically confirmed knee OA. The nine participants were divided into three dosage groups (low, medium or high). All participants received a single intra-articular injection of the gene transfer vector sc-rAAV2.5IL-1Ra. The study's primary outcome measures were safety and tolerability.
The researchers followed participants for a year, assessing transgene expression, as determined by IL-1Ra concentrations in synovial fluid, humoral and cell-mediated immune responses to the treatment, and patient-reported outcome measures of pain and function. Study participants underwent X-ray and MRI assessment of the index knee joints.
Results and conclusions
Study results shared in the journal publications included the following:
- There were no serious adverse events (AEs) related to the gene delivery treatment.
- Participants had elevated IL-1Ra concentrations in synovial fluid for a year after treatment.
- The treatment generated a humoral, but not a cell-mediated, immune response to the viral capsid.
- Patient-reported outcomes confirmed improvements in pain and function.
"This phase 1 clinical trial confirmed that local gene therapy in knee joints with osteoarthritis is safe and able to achieve a sustained, intra-articular concentration of a therapeutic gene product," explains Dr. Evans.
Although patient-reported outcomes confirmed improvements in pain and function to varying degrees, Dr. Evans and colleagues acknowledge that the validity of these findings is limited by the study size and the absence of a placebo group. Dr. Evans notes that successful phase 2 and 3 trials will be necessary before the treatment can achieve Food and Drug Administration (FDA) approval.
"If approved, the clinical impact will be substantial because, as noted above, the clinical management of osteoarthritis is presently very challenging," says Dr. Evans.
Next steps
Dr. Evans and colleagues are already pursuing the next milestone in their efforts to advance knowledge about this novel OA treatment. He notes: "We are focused on conducting the pivotal clinical trials necessary to confirm safety and efficacy in larger numbers of patients. To achieve this, we have established an arthritis gene therapy company that has already completed a successful phase 1b study involving over 60 patients." The company is also in discussions with the FDA about the structure of future phase 2 and 3 trials.
Dr. Evans holds joint appointments in Physical Medicine and Rehabilitation, Orthopedic Surgery, and Molecular Medicine at Mayo Clinic in Rochester, Minnesota.
For more information
Musculoskeletal Gene Therapy Research Laboratory: Christopher H. Evans. Mayo Clinic.
De la Vega R, et al. A phase 1 clinical trial shows safe, sustained, AAV-mediated expression of IL-1Ra in the human osteoarthritic knee joint. Science Translational Medicine. 2025;17:eadu9804.
Evans CH, et al. Osteoarthritis gene therapy: Expanding the scope of genetic therapies. Molecular Therapy. 2025;33:3456.
Refer a patient to Mayo Clinic.