Feb. 01, 2019
The first 50 years
There were three major revolutionary innovations and paradigm shifts in congenital heart disease (CHD) interventions over the last half-century. These innovations were the heart-lung (cardiopulmonary bypass) machine, neonatal cardiac surgery and transcatheter therapy.
Heart-lung machine
The advent of the heart-lung machine in the mid-1950s marked the birth of cardiac surgery and the genesis of CHD as a subspecialty. Prior to this, there had been several successful extracardiac CHD interventions such as the first ligation of patent ductus arteriosus by Dr. Robert Edward Gross in 1938, repair of coarctation of the aorta by Dr. Clarence Crafoord in 1944, subclavian to pulmonary artery (Blalock-Taussig) shunt performed by Dr. Alfred Blalock in 1944, and the first pulmonary artery banding by Dr. William H. Muller Jr. in 1951.
Dr. F. John Lewis performed the first intracardiac surgical procedure in 1952 when he repaired an atrial septal defect using moderate hypothermia and the caval inflow occlusion technique. This was immediately followed by repair of several moderately complex CHD diagnoses such as tetralogy of Fallot and complete atrioventricular septal defect using the cross circulation technique that was pioneered by Dr. C. Walton Lillehei. In spite of these early successes, the need to subject a healthy donor to the risks of anesthesia, anticoagulation and cannulation with the added risk of possible air embolism made the cross circulation technique less than practical for routine use.
首创的 Mayo-Gibbon 泵式氧合器
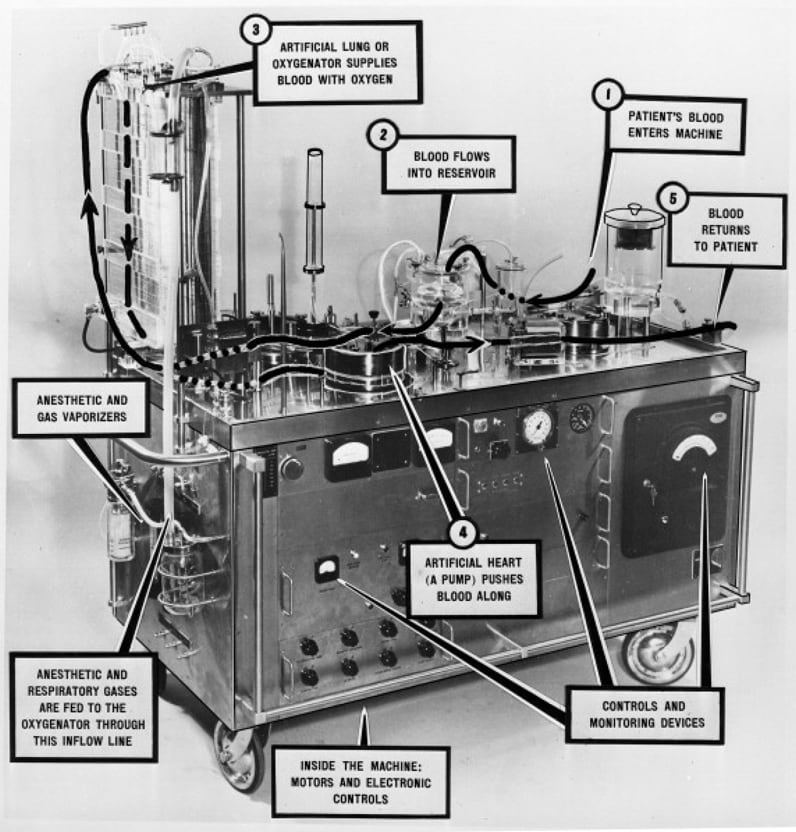
首创的 Mayo-Gibbon 泵式氧合器
首创的 Mayo-Gibbon 泵式氧合器,在妙佑医疗国际明尼苏达州罗切斯特院区的妙佑医疗国际历史展览会上展出。
The first successful application of the heart-lung machine was by Dr. John Gibbon Jr. when he repaired an atrial septal defect in a young woman in 1953. Unfortunately, subsequent attempts to replicate this operation all resulted in surgical mortality over the next two years. However, the tides turned when Dr. John Kirklin used a modification of this heart-lung machine (Mayo-Gibbon pump-oxygenator) in a series open-heart operations from 1955 with some success. Subsequent modifications of the heart-lung machine such as the DeWall bubble oxygenator, which was cheap and easy to assemble, catalyzed the proliferation of open-heart surgical centers in the United States and throughout the world. The next decade witnessed the emergence of new surgical techniques for more-complex lesions such as the Senning operation in 1957, Mustard operation in 1963 and the Fontan operation in 1968.
The arterial switch operation
The arterial switch operation was first introduced by Dr. William Mustard in Toronto in 1952 although the patient did not survive. The first successful switch was performed by Dr. Adib Jatene of Brazil in a 42-day-old infant with transposition of the great arteries and ventricular septal defect in 1975. This marked the beginning of an era of early primary repair of complex congenital heart defects.
Neonatal cardiac surgery
The next revolutionary innovation in CHD interventions was the advent of neonatal cardiac surgery. Prior to this, neonates and young infants with complex CHD had to undergo a palliative procedure as a bridge to corrective surgery later in life. In the late 1960s and early 1970s, Dr. T. Horiuchi and colleagues from Japan and Dr. Brian Barratt-Boyes from New Zealand were reporting good results with primary repair of tetralogy of Fallot in infants.
The Boston Children's Hospital followed this trend under the leadership of Dr. Aldo Castaneda and accumulated a large and satisfactory experience with open-heart operations in neonates and infants, including the first arterial switch operation in an 11-day-old neonate in 1983. Neonatal cardiac surgery changed the CHD landscape by reducing the need for staged surgical repair (palliative surgery followed by corrective surgery) and its associated morbidity and mortality, and increased the population of CHD patients that were eligible for heart surgery.
The first successful transcatheter cardiac procedures were balloon atrial septostomy by Dr. William Rashkind in 1966, patent ductus arteriosus closure using an Ivalon plug in 1967, and atrial septal defect closure by Dr. Terry Dean King in 1975. A new frontier in CHD interventions emerged after the first successful transcatheter aortic valve replacement in an inoperable patient by the French cardiologist Dr. Alain Cribier in 2002. This paved the way for the development of Melody transcatheter valve, which has become the workhorse of transcatheter pulmonary valve therapy for nearly a decade.
In addition to these revolutionary interventions, the field of congenital cardiology also witnessed several other important milestones, such as significant improvement in perioperative anesthesia and monitoring, neonatal and pediatric critical care, fetal echocardiography and interventions, and improved patient survival resulting in the emergence of the adult congenital heart disease subspecialty.
The next 50 years
Despite the landmark innovations of the first 50 years, the next 50 years appear even more promising. There are multiple evolving areas of expertise that will potentially shape the congenital cardiovascular specialty over the next half-century.
The first is the creation of the adult congenital heart disease subspecialty. With up to 90 percent of CHD patients surviving to adulthood in the current era, the next 50 years will likely see a significant increase in the prevalence of acquired heart disease in this population. Well-coordinated specialized multidisciplinary care from providers with skills in both pediatric and adult medicine will become critical for the prevention and management of acquired heart disease as well as the long-term sequelae of CHD in this population.
Second, one of the downstream problems of an aging population with CDH is an increase in the average number of surgical re-interventions in a lifetime, and the need for multiple re-operations in patients with complex anatomy will invariably result in an increase in surgical morbidity or mortality or both. Transcatheter valve therapy is now increasingly being used to mitigate or delay the need for multiple high-risk surgical re-operations. Although transcatheter valve therapy was an innovation of the first 50 years, the application of this therapy is significantly limited by the anatomic variability of the population with CDH making most patients ineligible candidates. The ongoing improvement in the design of valve prostheses and delivery systems specifically engineered to accommodate the anatomic variability of this population will potentially increase the number of patients eligible for this therapy in the next half-century.
Third, the advances in imaging techniques (CT, MRI, echocardiography) that include 3D imaging and the ability to print models and evaluate anatomy in a virtual environment allow a more thorough preparation for a complex intervention. In addition, the ability to practice the actual procedure in advance of the operation provides an effective model for education and may help reduce morbidity and mortality for complex or infrequently performed percutaneous or open surgical procedures.
Fourth, the demand for cardiac replacement therapy will continue to rise as the population of those with CDH ages and the prevalence of end-stage heart failure increases. Unfortunately, the limited availability of donor heart organs and pre-sensitization from prior operations have resulted in a huge mismatch between the supply of cardiac allografts and the rising demand due to end-stage heart failure. With the ongoing improvements in the newer generation of ventricular assist devices, Mayo specialists anticipate that the use of a ventricular assist device as destination therapy for patients with CHD who have end-stage heart failure may become the standard of care in the near future.
Lastly, stem cell therapy is an emerging field with many exciting ongoing research studies. The clinical application of stem cell therapy, though still at the preliminary stage, is very promising for slowing down the progression to end-stage heart disease or even potentially reversing myocyte damage from previous insult.
At the turn of the 20th century, complex CHD was uniformly fatal with no meaningful treatment options. The last 50 years have witnessed the birth and exponential growth of CHD interventions. More importantly, the next 50 years will likely see more revolutionary innovations in this field.