April 20, 2021
Age-related macular degeneration is the leading cause of blindness in the developed world. The disease, which causes a loss of central, high-acuity vision, is thought to result from dysfunction of the retinal pigment epithelium (RPE).
The concept of treating age-related macular degeneration using RPE transplantation has been studied since the late 1980s, but few clinical trials were conducted due to lack of an abundant RPE cell source. Today, pluripotent stem cells offer that source; studies have demonstrated the cells' reliable differentiation into RPE cells. "Since RPE cells are no longer a rate-limiting factor, the field has shifted focus toward development of a practical means of delivering RPE cells into the subretinal space," says Alan D. Marmorstein, Ph.D., with Ophthalmology Research at Mayo Clinic in Rochester, Minnesota.
Caracterización de un implante de hidrogel de fibrina
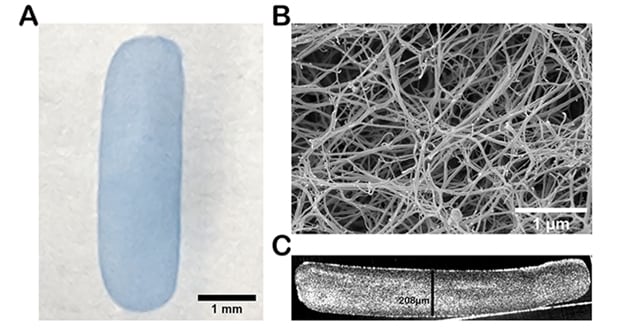
Caracterización de un implante de hidrogel de fibrina
A. Una imagen de campo claro de un implante de fibrina de 1,5 mm × 5,0 mm teñido con azul de tripano. B. La micrografía por barrido electrónico de la superficie superior del implante de fibrina muestra los característicos hilos como las fibras entrecruzadas. C. La tomografía de coherencia óptica del implante de fibrina en sección transversal muestra la formación y el grosor uniformes de la fibrina.
In an animal study published in PLOS One in 2020, Dr. Marmorstein and fellow researchers report the following about surgically implanted fibrin scaffold placed in the subretinal space:
- The scaffold consistently degraded within eight weeks without damage to the neurosensory retina or endogenous RPE.
- After the scaffold was degraded, the retina appeared to reattach to the underlying RPE.
- When the RPE was mechanically debrided, the implant was degraded within four weeks.
"To the best of our knowledge, this is the first report that demonstrates complete degradation of a scaffold within the subretinal space," says Dr. Marmorstein.
Implantation device prototype development
To implant the fibrin scaffolds, the research team developed a novel insertion device with a rectangular housing tip geometrically optimized to house the implant. The outer dimensions of the tip were minimized to facilitate a small sclerotomy.
"To minimize hand-placement instability, we used a pneumatic-driven actuator that did not require finger manipulation, allowing compatibility with the viscous fluid injection module of common surgical vitrectomy systems, which can be triggered by a foot pedal," says Dr. Marmorstein.
A rectangular, transparent housing tip allowed visualization of the implant, which permitted the surgeon to verify proper polarity of the implant and deployment without damage to the implant. The stainless steel hub was curved to parallel the inner surface of the eye.
The research team chose a surgical model compatible with current vitreoretinal surgical practice: It included a transvitreal, transretinal approach to gain access to the subretinal space through the creation of a retinal detachment and retinotomy following vitrectomy.
Successful placement of the fibrin implant into the area centralis was achieved in 16 animals. Three of these surgeries included mechanical debridement of the RPE. Twelve surgeries had fluid-air exchange, one received a silicone oil tamponade and two received approximately 20% sulfur hexafluoride (SF6) gas tamponade. Results included:
- In cases with only fluid-air exchange, the air resorbed by day two or three postoperative.
- In cases with 20% SF6 gas tamponade, the gas resorbed completely by day five postoperative. In cases with partial gas in the globe, imaging or a full indirect exam were not possible.
- In the case with the silicone oil tamponade, both optical coherence tomography (OCT) and fundus images were difficult to obtain, and indirect exam was the preferred method of examination.
When fibrin scaffolds were visualized with indirect ophthalmoscopy and OCT at one week, researchers observed a rectangular gap between the retina and underlying choroid-Bruch's membrane-RPE tissue that was later confirmed by histology as the fibrin implant.
Of the 13 animals receiving fibrin scaffold implants in which RPE was not debrided, all showed signs of fibrin degradation over time. OCT images showed a raised plateau that completely resolved by week eight without damage to the retina or choroid-Bruch's membrane-RPE tissue complex.
RPE debridement accelerates fibrin degradation
To test the hypothesis that removal of the native RPE may accelerate fibrin degradation, the research team mechanically debrided a region of the endogenous RPE in two animals. Over time, the fibrin implant degraded at a faster pace. Histology at four weeks confirmed no evidence of the implant.
"Our data demonstrate that fibrin is a degradable, safe material for subretinal transplantation. Fibrin implants degraded within eight weeks of implantation with no signs of damage to the neural retina or underlying choroid-Bruch's membrane-RPE tissue complex, and debridement of the native RPE accelerated the degradation to four weeks post-implantation," says Dr. Marmorstein.
For more information
Gandhi JK, et al. Fibrin hydrogels are safe, degradable scaffolds for subretinal implantation. PLOS One. 2020;15:e0227641.