Nov. 25, 2021
Mayo Clinic has demonstrated that a slow-release local anesthetic can improve pain control after surgery for Chiari malformation type 1. A single intraoperative dose of liposomal bupivacaine can reduce the use of opioids in pediatric and young adult patients in the first 24 hours after surgery.
"The Chiari operation is one of the more painful operations that we offer for pediatric neurosurgery. We decided to address that and make it a better tolerated procedure," says Edward S. Ahn, M.D., a pediatric neurosurgeon at Mayo Clinic in Rochester, Minnesota.
Chiari malformation type 1 is common, with a natural incidence as high as 1% in children. Surgical decompression reduces symptoms, but the required muscle dissection causes significant pain, particularly in the first 24 hours after surgery. Oral opioids are typically administered, and there is a known risk of persistent opioid use after surgery.
Although liposomal bupivacaine has been used to treat adults after spinal surgery, the safety and efficacy of this anesthetic in children after Chiari decompression surgery hasn't been explored.
Control del dolor intraoperatorio
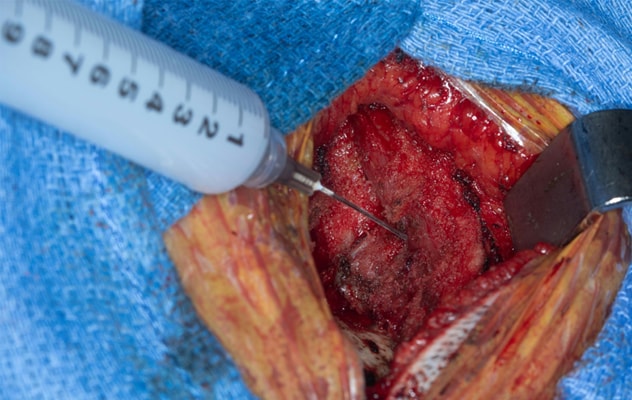
Control del dolor intraoperatorio
La foto muestra la administración intraoperatoria de bupivacaína liposomal en el músculo al final de la cirugía por malformación de Chiari tipo I antes del cierre facial. Imagen reimpresa con permiso de Journal of Neurosurgery: Pediatrics (Revista de Neurocirugía: Pediatría).
In a retrospective review published in the January 2021 issue of the Journal of Neurosurgery: Pediatrics, Mayo Clinic researchers found that patients given an intraoperative injection of liposomal bupivacaine had significantly lower opioid use and mean pain scores in the first 24 hours after surgery compared with patients who didn't receive that anesthetic.
In the period from 24 hours after surgery until hospital discharge, pain levels and opioid use in the two groups were comparable. Mayo Clinic has since instituted a new protocol that uses nonopioid medications to aggressively treat pain during that time.
Dr. Ahn notes that two patients in the study didn't require any postoperative opioids. "We continue to find that some patients who have liposomal bupivacaine don't need opioids after surgery," he says. "In general, we're finding that the combination of the intraoperative injection plus the new postoperative protocol is very positive. We're seeing patients recover much more quickly."
The value of cross-talk
Mayo Clinic's study of liposomal bupivacaine during Chiari malformation type 1 surgery included all patients who had the procedure between 2018 and 2020. The mean age of the patients was 15.9 years. Among the 18 patients in the study, half had liposomal bupivacaine and half did not.
No surgical complications were observed over a mean length of hospital stay of 2.7 days. To avoid complications, Mayo Clinic neurosurgeons confirm the presence of a solid dura barrier between the injection site and the patient's central nervous system before injecting liposomal bupivacaine. That precaution helps avoid exposing the nervous system to the anesthetic.
The study's finding of comparable pain levels and opioid use beginning 24 hours after surgery indicates the possible limit of the intraoperative anesthetic's effectiveness. "After the initial postoperative period, we supplement with nonopioid adjuncts," Dr. Ahn says.
Those nonopioid adjuncts are benzodiazepines and nonsteroidal anti-inflammatory medications, given on a scheduled basis. Previously, pain medications after Chiari malformation type 1 surgery were given as needed.
The use of intraoperative liposomal bupivacaine in the procedures was suggested by Dawit T. Haile, M.D., an anesthesiologist in the Mayo Clinic Children's Center who observed the pain experienced after Chiari malformation type 1 surgery. "I didn't know about this agent, but Dr. Haile knew it was being used in other disciplines," Dr. Ahn says.
"That's the spirit of Mayo Clinic," Dr. Ahn says. "We are open to finding ways to improve the patient experience. The cross-talk we have among specialties makes that possible."
For more information
Lu VM, et al. Effects of intraoperative liposomal bupivacaine on pain control and opioid use after pediatric Chiari I malformation surgery: An initial experience. Journal of Neurosurgery: Pediatrics. 2021;27:9.