Aug. 11, 2020
Case
A 36-year-old woman with no significant past medical history presented for evaluation of multiple, nontraumatic fractures discovered during a work-up of diffuse bone pain a year prior to her presentation.
At that time, she had developed progressive bilateral hip pain that was thought to be related to sacroiliitis. She subsequently underwent an extensive rheumatologic work-up that did not identify any inflammatory disorder to explain her symptoms. Radiographic imaging obtained did, however, reveal multiple fractures, including multiple rib fractures, a humeral fracture and sacral fractures. An oncologic work-up that included multiple bone marrow biopsies did not identify any malignancy.
The patient did not recall any prior fractures. She was healthy as a child and reached expected midparental height during puberty. She had no significant dental issues, nor did her first-degree relatives. Her maternal grandfather developed rickets at age 6, which resolved with appropriate therapy.
Patient laboratory testing results
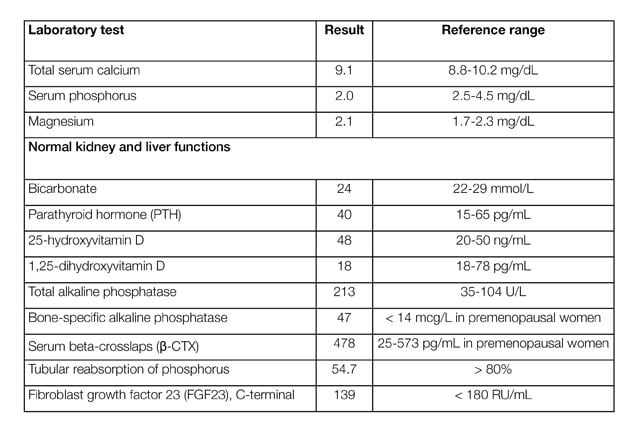
Patient laboratory testing results
Increased radiotracer uptake
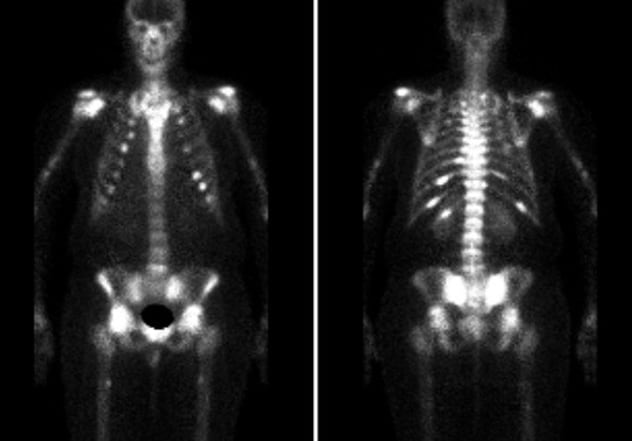
Increased radiotracer uptake
Increased radiotracer uptake of the bilateral sacroiliac region, posterior ninth to 12th ribs on the left, posterior 11th rib on the right, bilateral humeral heads, femoral heads, and ankles. Stress fractures of bilateral femurs and tibia.
Improvement of multiple fractures
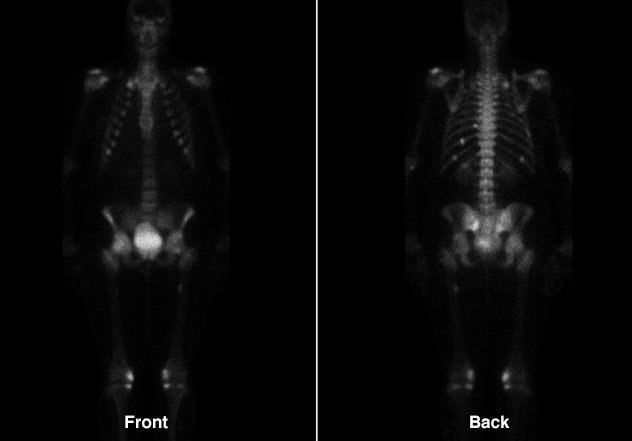
Improvement of multiple fractures
Improvement of multiple fractures involving ribs, sacrum and right proximal femur.
Examination did not identify any dental abnormalities or other dysmorphic features. The patient used a wheelchair due to multifocal pain. She underwent various laboratory tests.
Computerized tomography (CT) of the chest, abdomen and pelvis revealed a 6-cm cavernous hemangioma of the liver, which the patient has been aware of for at least six years. Technetium-99m (Tc99m) bone scintigraphy showed increased radiotracer uptake concerning for bilateral sacroiliac insufficiency fractures in addition to posterior left ninth to 12th and right 11th rib fractures. In addition, there were focal areas of uptake in bilateral humeral heads, femoral heads, femurs, tibia and ankles, all consistent with occult fractures. Whole-body magnetic resonance imaging (MRI), whole-body Tc99m sestamibi scan and a 68Ga-DOTATATE positron emission tomography (PET)-CT did not reveal any evidence of a mesenchymal tumor. In situ hybridization studies for fibroblast growth factor 23 (FGF23) were performed on a biopsy specimen obtained from the liver hemangioma and were negative.
The patient was started on calcitriol (1 mcg twice daily) and phosphorus (750 mg three times daily) for medical management of oncogenic osteomalacia. Within eight weeks of initiating medical therapy, she had a significant decline in bone pain, was able to wean off narcotic pain medications and was ambulating freely. At one-year follow-up, serum phosphorus levels were normal and repeat Tc99m bone scintigraphy showed healing of the previously noted insufficiency fractures. The patient had symptomatically improved, but continued to have a high medication burden.
Discussion
Oncogenic osteomalacia — also referred to as tumor-induced osteomalacia (TIO) — is a rare endocrine disorder in which a small bony or soft tissue mesenchymal tumor causes hypophosphatemia via secretion of FGF23. The latter causes hypophosphatemia via two mechanisms: 1) reduction of renal tubular phosphate reabsorption leading to phosphaturia, and 2) impairment of hydroxylation of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, thus reducing intestinal phosphorus absorption. As a result of chronic hypophosphatemia, patients develop osteomalacia and associated insufficiency fractures.
Testing considerations in oncogenic osteomalacia
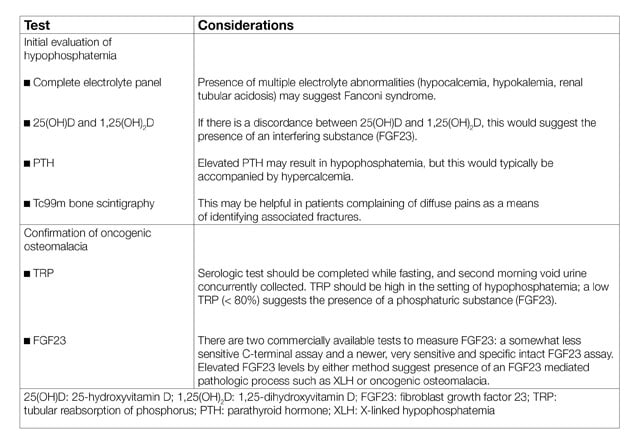
Testing considerations in oncogenic osteomalacia
The diagnosis of TIO should be considered in patients who have musculoskeletal pain with hypophosphatemia, with or without insufficiency fractures. Diagnostic testing should include quantification of the tubular reabsorption of phosphorus, which is reduced in the presence of FGF23; additional diagnostic laboratory and imaging studies are useful for the evaluation of TIO . The differential diagnosis of hypophosphatemia includes X-linked hypophosphatemia (consider with younger age of onset, suggestive family history and dental anomalies), proximal renal tubulopathies (consider with multiple electrolyte abnormalities; may be genetic or acquired), and dietary-related hypophosphatemia (consider in patients with low intake or refeeding syndrome). An endocrinologist's perspective of this condition was reviewed in the Journal of Endocrinological Investigation in 2018.
When TIO is biochemically confirmed, diagnostic imaging may help identify the culprit lesion; excision of the tumor can normalize FGF23 levels and reverse the disease process. Functional imaging such as 18F-fluorodeoxyglucose (FDG) PET-CT and octreotide scintigraphy with single-photon emission computerized tomography (SPECT)-CT have been utilized with some success in identification of these lesions. Anatomical imaging with MRI (whole body or focused on area with positive functional imaging) may be considered. Venous sampling for measurement of FGF23 has been used with variable success. While not approved by the Food and Drug Administration (FDA) for mesenchymal tumors, 68Ga-DOTATATE PET-CT has been reported in Clinical Nuclear Medicine in 2015 to have some success in tumor identification; a current clinical trial (NCT03736564) is investigating its use for this indication.
Tumor identification is commonly unsuccessful early on in the disease process; supplementation with phosphorus and calcitriol is the mainstay of medical management. Doses should be escalated, as tolerated, until normalization of serum phosphorus levels. Serial monitoring is necessary because of long-term risks of nephrocalcinosis and secondary hyperparathyroidism, as well as changes in requirements for phosphorus. Imaging studies can be repeated at regular intervals in an effort to identify the culprit lesion and avoid long-term medical management. In June 2020, the FDA approved an FGF23 antibody, burosumab, for the treatment of oncogenic osteomalacia; in addition to improving phosphate metabolism and physical function, this novel therapy may reduce the burden of treatment and monitoring for this difficult condition.
For additional information
Boland JM, et al. Phosphaturic mesenchymal tumors: What an endocrinologist should know. Journal of Endocrinological Investigation. 2018;41:1173.
Agrawal K, et al. Comparison of 18F-FDG and 68Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clinical Nuclear Medicine. 2015;40:e6.
Clinical trials: Ga-DOTATATE PET for Localization of Phosphaturic Mesenchymal Tumors in Patients with Tumor Induced Osteomalacia. Mayo Clinic.
FDA approves first therapy for rare disease that causes low phosphate blood levels, bone softening. U.S. Food and Drug Administration.