Nov. 21, 2014
Severe, untreated mitral regurgitation (MR) leads to progressive left ventricular (LV) dysfunction and heart failure, with mortality for symptomatic patients of at least 5 percent annually. Aggressive medical management may attenuate symptoms but does not change mortality figures, says Vuyisile T. Nkomo, M.D., co-director of the Valvular Heart Disease Clinic at Mayo Clinic in Rochester, Minnesota. The only definitive treatment is operative, with mitral valve repair or replacement.
American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend surgery for moderate-to-severe or severe MR in symptomatic patients and those with evidence of LV dysfunction. Unfortunately, many of the most symptomatic patients are elderly and medically fragile and not candidates for surgical repair. The recent ACC/AHA 2014 guidelines now recommend transcatheter mitral valve repair for individuals with primary or degenerative MR and prohibitive surgical risk.
The early percutaneous approach to mitral valve disease focused on the treatment of mitral stenosis with balloon valvuloplasty; more recently, paravalvular leaks have been closed with percutaneous occlusive devices. Percutaneous correction of MR has been more challenging. "Current procedures target leaflet modification; various percutaneous annuloplasty approaches are under investigation, but most are in early stages of development," according to Guy S. Reeder, M.D., interventional cardiologist at Mayo Clinic's campus in Minnesota. "Treatment of MR with percutaneous coronary sinus annuloplasty is intuitively attractive, but there are technical limitations."
The anatomic relationship of the coronary sinus to the valve annulus is highly variable, and the coronary sinus frequently runs along the atrial side rather than in the true plane of the annulus. "In more than half of patients, branches of the circumflex artery run beneath the coronary sinus; tightening a percutaneous annuloplasty ring in the coronary sinus may compress the circumflex artery, inducing myocardial ischemia or infarction," says Mackram F. Eleid, M.D., interventional cardiology fellow at Mayo Clinic's campus in Minnesota. "Preprocedural imaging studies have not reliably defined the relationship between the mitral valve annulus, the circumflex artery and the coronary sinus, nor have they predicted clinical outcomes. Also, it is not known how coronary sinus annuloplasty devices affect the use of coronary sinus leads for biventricular pacing."
Percutaneous leaflet modification has been more promising. Two percutaneous devices have been developed; conceptually, both techniques were derived from the Alfieri surgical repair, in which a mid-leaflet suture creates a double orifice valve. One device used a transseptal suction catheter to approximate the mitral leaflets and deploy cutaneous sutures. Trials demonstrated feasibility, but this approach was abandoned as the procedure was technically difficult.
TEE view of severe mitral valve regurgitation

TEE view of severe mitral valve regurgitation
Four-chamber TEE view demonstrating severe mitral valve regurgitation due to a flail P2 scallop with an eccentric posteromedially directed jet.
Mild residual MR
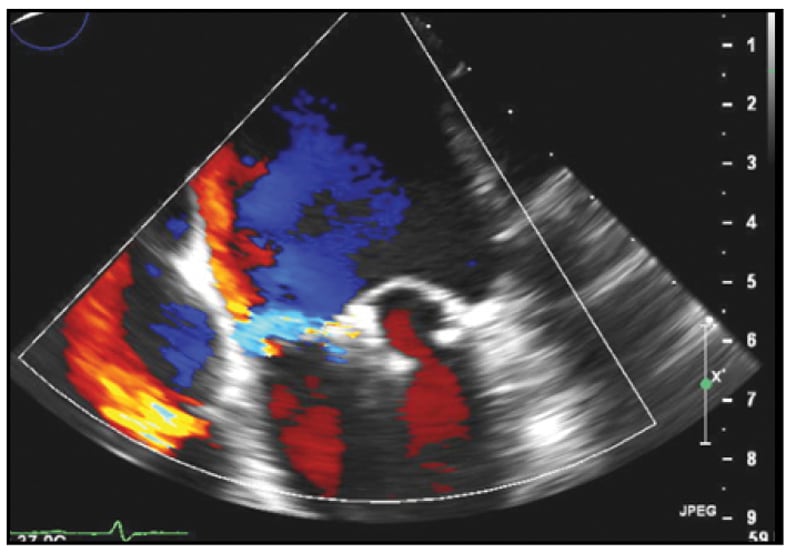
Mild residual MR
Following percutaneous placement of two adjacent MitraClip devices, mild residual MR is present.
Two MitraClip devices
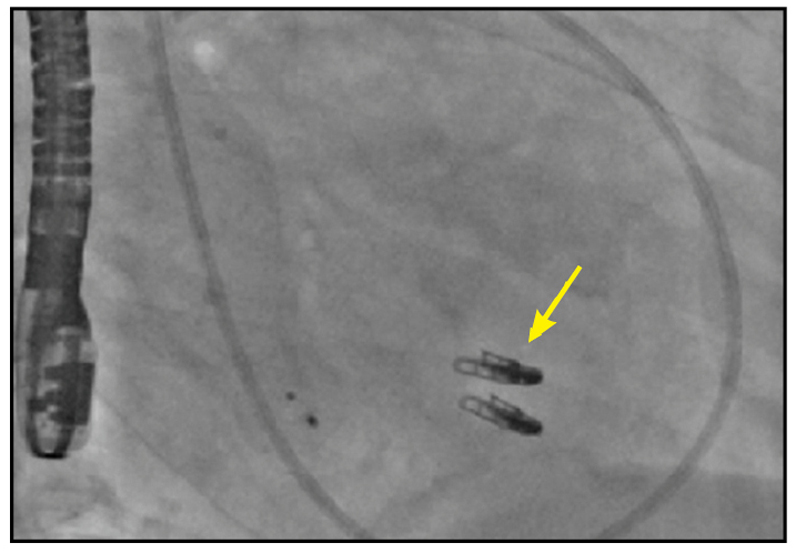
Two MitraClip devices
Right anterior oblique cinefluoroscopic view demonstrating two MitraClip devices (yellow arrow).
The current approach to percutaneous leaflet modification uses the MitraClip device (Abbott Vascular, Santa Clara, California), which fixes or "clips" the anterior and posterior mitral leaflets together under transesophageal echocardiographic (TEE) guidance. The MitraClip device is 4 millimeters (mm) wide and made of cobalt-chromium; its two arms or clips are opened and closed by a handle on the delivery catheter. Like the Alfieri procedures, a double-orifice valve is created.
Importantly, the clip can be surgically removed and does not preclude later surgical treatment if warranted. Animal studies suggest that the clip becomes encapsulated within three months after implantation. MitraClip has been CE mark approved for clinical use in Europe since 2005, where more than 8,000 patients have been treated in the past decade.
EVEREST I and EVEREST II
The Endovascular Valve Edge-to-Edge Repair Trials (EVEREST I and EVEREST II) evaluated the safety and efficacy of the MitraClip in high-risk patients with severe MR. EVEREST I was a feasibility trial; 55 patients were enrolled and 49 received the device. EVEREST II evaluated patients from 37 centers in Canada and the United States. Patients with MR were symptomatic and had LV ejection fraction greater than 25 percent and LV end-systolic diameter of 55 mm or less. Asymptomatic patients had to have LV ejection fraction of 25 to 60 percent, LV end-systolic diameter of 40 to 55 mm, new atrial fibrillation or pulmonary hypertension to enroll.
A total of 279 patients were randomly assigned in a 2-1 ratio to receive the MitraClip versus standard surgery. The primary composite end point was freedom from death, subsequent surgery for MR, and grade 3+ or 4+ MR at one year. Researchers found that the 30-day mortality rate for MitraClip was much lower than that for surgery (4.8 vs. 18.2 percent), with a 96 percent implant success rate and a low rate of adverse events.
Importantly, patients identified major improvements in quality of life; also noted was a significant reduction in LV size and hospitalization rates during the first year after MitraClip placement compared with the prior year. However, 20 percent of individuals who received the MitraClip required operative repair of the valve within the first year because of persistent or recurrent MR, compared with 2.2 percent in the surgery group.
The ongoing EVEREST registry and the European experience may provide data on the long-term durability of MitraClip repair. The MitraClip is approved by the Food and Drug Administration to treat patients with severe symptomatic primary, degenerative MR who would be too high risk for surgery. For patients with severe secondary, functional MR, the MitraClip is available through participation in the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial, in which patients are randomly assigned to receive the device or medical therapy.
At Mayo Clinic, candidates for MitraClip are evaluated by a multidisciplinary team of imaging specialists, interventionalists and cardiac surgeons; there is no pre-specified Society of Thoracic Surgeons (STS) threshold for risk. Standard evaluation of valve suitability involves TEE assessment of the regurgitant mechanism and location and the technical suitability of valve morphology, including leaflet coaptation length.
Mitral valve before and after repair

Mitral valve before and after repair
3D TEE surgeon's view of the mitral valve before (top) and after (bottom) repair, resulting in a double orifice mitral valve.
The procedure is performed in the cardiac catheterization laboratory under general anesthesia using TEE guidance. Access is percutaneous via the right femoral vein, with most patients discharged on post-procedure day one or two. Patient experience with the procedure has been favorable, with minimal access site discomfort and rapid recovery.
For more information
For more information about MitraClip protocols at Mayo Clinic, contact the Valvular Heart Disease Clinic at Mayo Clinic in Rochester at 507-266-4044.
Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation. ClinicalTrials.gov.