Feb. 09, 2018
Malignancies before and after immunotherapy
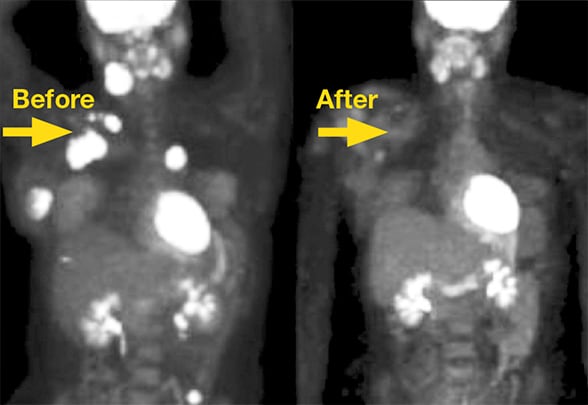
Malignancies before and after immunotherapy
Malignancies before and after immunotherapy using immune checkpoint inhibitors.
Over the last few years, immunotherapy using immune checkpoint inhibitors targeting programmed cell death receptor 1 (PD-1) or programmed cell death receptor ligand 1 (PD-L1) has revolutionized the therapeutic approach to advanced-stage non-small cell lung cancer and numerous other malignancies.
Monotherapy, using the anti-PD-1 antibody pembrolizumab, is now Food and Drug Administration (FDA) approved for patients with stage IV non-small cell lung cancer without a driver mutation and 50 percent or greater tumor cell PD-L1 expression by immunohistochemistry staining. Moreover, the combination of systemic platinum-based chemotherapy and pembrolizumab was also recently approved for first line therapy of metastatic nonsquamous non-small cell lung cancer independent of PD-L1 status.
Nivolumab (anti-PD-1) and atezolizumab (anti-PD-L1) also are FDA approved for second line therapy of stage IV lung cancer. For this indication, with the exception of pembrolizumab, which requires 1 percent or greater PD-L1 expression, no PD-L1 expression level is required to initiate therapy.
On average, objective response rates to immunotherapy range around 20 to 30 percent and sustained long-term responses have been observed. However, since the majority of patients do not benefit from second line immunotherapy, many efforts are being made to improve durable responses. The newest addition is a Mayo Clinic investigator-initiated study, the Trial of Measles Virotherapy in Combination With Atezolizumab in Patients With Metastatic Non-Small Cell Lung Cancer, which explores the combination of the intratumoral administration of the measles virus with systemic PD-L1 targeted immunotherapy using atezolizumab for second line therapy of non-small cell lung cancer.
Immune-mediated adverse events
PD-1/PD-L1 targeted immunotherapy is generally well-tolerated and has fewer acute side effects compared with standard cytotoxic chemotherapies. However, these new therapeutic agents have been linked to a growing number of immune-mediated adverse events (imAEs).
Immune-mediated adverse events
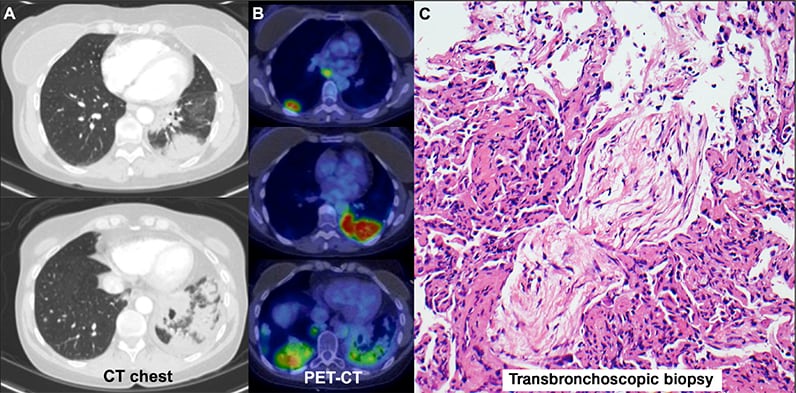
Immune-mediated adverse events
CT chest scan, PET-CT scan and transbronchoscopic biopsy indicating immune-mediated adverse events.
ImAEs are observed in 70 percent of patients and include autoimmune colitis, thyroiditis, hypophysitis, dermatitis and pneumonitis. The severity of imAEs ranges from asymptomatic to life-threatening presentations, while the time course can be either transient or chronic and relapsing.
Mayo Clinic's true multidisciplinary team approach includes experts in medical oncology, rheumatology, pulmonary medicine, gastroenterology, endocrinology and neurology to optimally manage patients with imAEs. Besides stopping immunotherapy, patients are most commonly treated with immunosuppression (most commonly corticosteroids).
The pathogenesis, risk factors, diagnostic criteria and individualized management of patients experiencing immunotherapy-related imAEs outside of controlled clinical trials, however, remains unclear. A diagnosis of an imAE, published in Melanoma Research in 2016, represents a diagnosis of exclusion and depends on the active elimination of alternative diagnosis.
For more information
Vyriad. Trial of Measles Virotherapy in Combination With Atezolizumab in Patients With Metastatic Non-Small Cell Lung Cancer. ClinicalTrials.gov.
Kottschade L, et al. A multidisciplinary approach to toxicity management of modern immune checkpoint inhibitors in cancer therapy. Melanoma Research. 2016;26:469.