Sept. 27, 2023
Hematopoietic stem cell transplantation (HCT) is a potentially curative and lifesaving treatment option for hematologic cancers such as leukemia, lymphoma and myeloma. Since the first HCT was performed in 1956, the growth of this therapy has been exponential with almost 100,000 HCTs performed annually worldwide. During HCT, patients undergo high-dose chemotherapy, radiation therapy or both with a view to eradicate any cancer cells. Since the dose of chemotherapy is so high, the patient's bone marrow is eradicated at the same time, and without transplant the patient has no ability to produce red blood cells, platelets or white blood cells.
Stem cells are collected beforehand and are administered after conditioning is complete to reconstitute the immune system. HCT may use the patient's own stem cells (autologous) or stem cells from an appropriately matched donor (allogeneic). "Historically, HCT has been considered very high risk," says Hemang Yadav, M.B.B.S., a pulmonologist at Mayo Clinic in Rochester, Minnesota. "However, in the last 20 years, advances in management of peri-transplant infection, treatment of graft-versus-host disease and better donor-recipient matching has resulted in a dramatic improvement in the survivorship of the HCT recipient."
造血干细胞移植后肺部并发症的时程
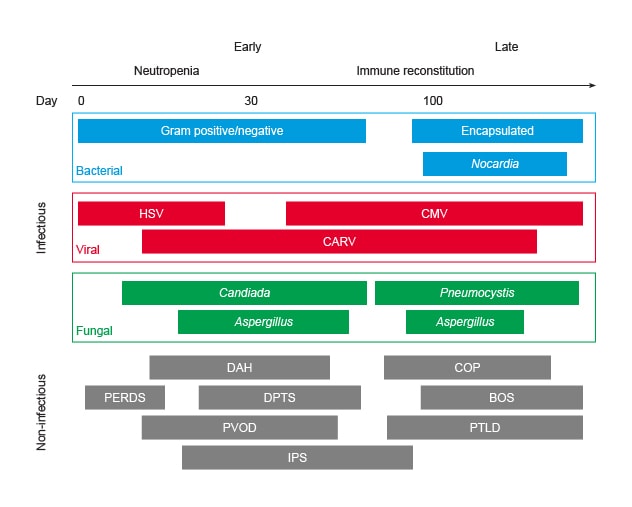
造血干细胞移植后肺部并发症的时程
BOS:闭塞性细支气管炎综合征;CARV:社区获得性呼吸道病毒;CMV:巨细胞病毒;COP:隐源性机化性肺炎;DAH:弥漫性肺泡出血;DPTS:迟发性肺毒性综合征;HSV:单纯疱疹病毒;IPS:特发性肺炎综合征;PERDS:移植周围呼吸窘迫综合征;PTLD:移植后淋巴细胞增生性疾病;PVOD:肺静脉闭塞病。
图表经《世界重症监护医学杂志》许可转载。
Pulmonary complications occur in one-third of recipients with HCT and are the most common cause of nonrelapse mortality after HCT, representing a major threat to the survivorship of the HCT recipient.
While other aspects of post-HCT care have improved, there has been little progress in understanding and management of HCT-related pulmonary syndromes. These complications can broadly be divided into those that have an infectious cause, for example, bacterial pneumonia and influenza pneumonia, and those that fall into one of the noninfectious lung injury syndromes. The latter include diseases such as peri-engraftment respiratory distress syndrome (PERDS), diffuse alveolar hemorrhage (DAH), idiopathic pneumonia syndrome (IPS) and others. The most severe end of this spectrum is acute respiratory distress syndrome (ARDS), which occurs in 5% of patients after HCT, with a mortality exceeding 60%.
"These syndromes are poorly understood, often clinically indistinguishable from each other and with overlapping pathophysiology," says Dr. Yadav. "The goal of our research at Mayo Clinic is to better understand why certain patients develop respiratory failure after HCT and study the mechanisms for how these diseases develop." With that understanding, physicians can more meaningfully identify the pathophysiology of respiratory failure for individual patients and use that understanding to develop methods for both early disease detection and disease prevention.
To try and determine potential causal factors that underly the development of post-HCT respiratory failure, detailed analyses was conducted in the pre-transplant, post-transplant and in-hospital domains. Study results were published in the Annals of the American Thoracic Society in 2021 and in Respiratory Care in 2023, respectively. These studies identified several risk factors unique to the post-HCT population.
Mayo Clinic has also recently completed enrollment for a large multicenter study, funded by the National Institutes of Health, whose goal is to develop and validate tools to better predict which patients will be at highest risk of lung complications following bone marrow transplantation.
These studies will allow us to identify the highest risk patients to then enroll in a digital remote monitoring program. This program, developed concurrently with the Center for Digital Health at Mayo Clinic, will enroll these high-risk patients after HCT and monitor vital signs including respiratory rate, oxygen saturation, as well as lung function through integrated home spirometry.
This monitoring will allow earlier detection of pulmonary disease in high-risk patients. When these high-risk patients are hospitalized after HCT, blood samples are collected regularly. Those patients who develop pulmonary complications will be compared with those who do not. This allows a better understanding of why some patients develop respiratory complications and some do not. "Our analysis approach leverages the emerging science of multiomics where we integrate immunology with proteomics and metabolomics to develop a holistic assessment of disease development," says Dr. Yadav.
Mayo Clinic's work will provide a better understanding of why patients develop pulmonary complications after HCT, develop tools to identify high-risk patients and enroll these high-risk patients in close post-HCT monitoring programs. "Eventually, the goal is to develop novel therapies, individualized to patient pathophysiology, that reduce the frequency and severity of pulmonary complications after HCT," says Dr. Yadav.
For more information
Herasevich S, et al. Pretransplant risk factors can predict development of acute respiratory distress syndrome after hematopoietic stem cell transplantation. Annals of the American Thoracic Society. 2021;18:1004.
Herasevich S, et al. Post-transplant and in-hospital risk factors for ARDS after hematopoietic stem cell transplantation. Respiratory Care. 2023;68:77.
Refer a patient to Mayo Clinic.