Aug. 30, 2019
Mayo Clinic is at the forefront of imaging innovations that are upending neurological care. Aligned with the enterprise's commitment to evidence-based medicine, these innovations apply quantifiable methods to neuroimaging, for individualized prognosis and treatment of brain tumors and epilepsy.
"This is going to revolutionize how we practice neuroimaging. The benefits for patients will be phenomenal," says Bradley J. Erickson, M.D., Ph.D., director of the Radiology Informatics Laboratory at Mayo Clinic in Rochester, Minnesota.
دماغ بشري مصاب بالورم الأرومي الدبقي
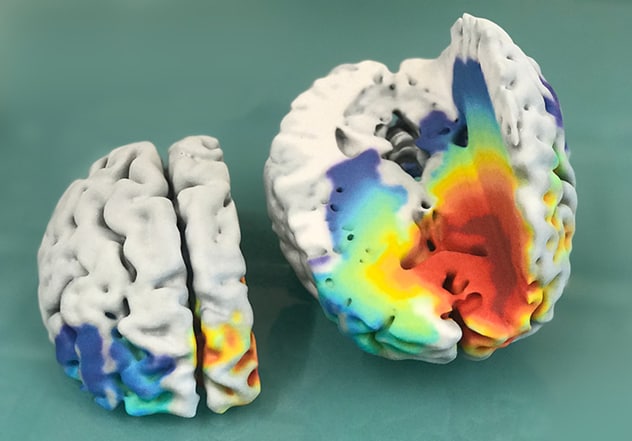
دماغ بشري مصاب بالورم الأرومي الدبقي
تُظهر الصورة نموذجًا لدماغ بشري مصاب بالورم الأرومي الدبقي، مطبوع من فحص تصوير بالرنين المغناطيسي ثلاثي الأبعاد، وتنبؤات النماذج الرياضية التي تبين كثافة مناطق خلايا الورم المتوقعة التي تتراوح بين الأقل كثافة (باللون الأزرق) والأكثر كثافة (باللون الأحمر الغامق). في العادة، تظهر المناطق الحمراء الغامقة فقط في صورة الرنين المغناطيسي.
The data-centered approach includes:
- Applying artificial intelligence to extract maximum information from MRIs of brain tumors
- Building mathematical models to predict tumor progression
- Using sophisticated functional imaging to precisely locate seizure origin sites in the brain
"Imaging already drives what's done daily in the clinic. But clinicians may not have all the tools necessary to help navigate individualized decisions. The power of these quantifiable approaches is that they can be applied to individual patients in ways that would otherwise be unattainable," says Kristin R. Swanson, Ph.D., director of the Mathematical Neuro-Oncology Laboratory at Mayo Clinic in Phoenix/Scottsdale, Arizona.
Informatics to optimize cancer treatment
Applying modern machine learning methods to medical images has the potential to substantially improve brain tumor diagnosis, particularly prior to tissue-based diagnosis. In cases of glioma, an increasingly important aspect of diagnosis is the identification of molecular biomarkers, which can be used to group diffuse gliomas that have similar clinical behavior, response to therapy and outcome.
Mayo Clinic's Radiology Informatics Laboratory recently succeeded in identifying the IDH1, 1P19Q and TERT glioma markers from routine MRI data, with 90% to 95% accuracy. "That's far beyond what any human visual system can do," Dr. Erickson says. The laboratory's similar success identifying MGMT methylation status — another important glioma biomarker — was described in the October 2017 issue of the Journal of Digital Imaging.
Large data sets are used to train deep learning algorithms to predict genomic patterns in tumors. "These algorithms seem to be able to identify textures or other properties of images that can be subtler than what we can visually perceive," Dr. Erickson says.
تصوير بالرنين المغناطيسي لورم في الدماغ
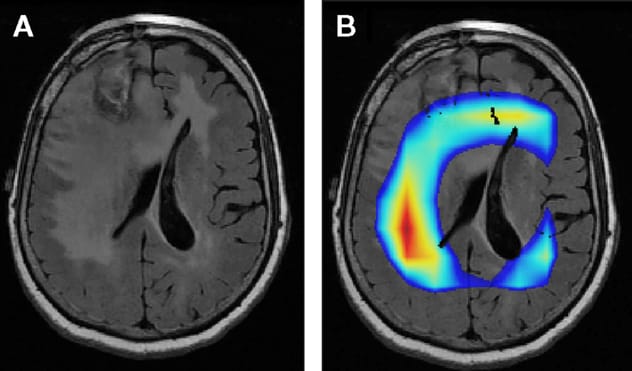
تصوير بالرنين المغناطيسي لورم في الدماغ
أ. تصوير بالرنين المغناطيسي يوضح ورمًا في الدماغ. ب. تشير المساحات الملونة إلى أجزاء الصورة التي تجدها خوارزمية Mayo Clinic مهمة لتحديد ما إذا كان الورم ناتجًا عن طفرة في إنزيم نازِعَةُ هيدرُوجين الإيزوسيترات أم لا. لا تتطابق المناطق الملونة مع المنطقة التي يظهر فيها مؤشر على وجود شيء غير طبيعي، الذي قد يعكس غزوًا مكرويًا كمؤشر على إنزيم نازِعَةُ هيدرُوجين الإيزوسيترات الطبيعي في مقابل الطافر.
Additional investigations focus on what this imaging can reveal about tumor biology. "We can tell that in some cases of IDH1 mutation, at least some of the MRI signal is coming from just outside the tumor margin. That's probably where tumor cell invasion is occurring," Dr. Erickson says. "Maybe those cells have a different physiology that affects how they resist the tumor invasion. The imaging may be reflecting something we don't yet understand about the tumor biology. Eventually, imaging may help us determine whether a patient will respond to a certain treatment."
Mathematical models are other tools in these efforts. Using large patient-centric data sets, the Mathematical Neuro-Oncology Laboratory combines predictive mathematical models and clinical oncology to predict patient-specific tumor growth and to investigate how various treatments affect tumors.
"We model what an individual's cancer will look like over time. Every patient has their own equation," Dr. Swanson says. "We can subsequently evaluate how far we've deflected a tumor off its growth curve, as a measure of treatment response."
Mathematical modeling is also shedding light on sex differences in glioblastoma outcomes. In a study published in the Jan. 2, 2019, issue of Sciences Translational Medicine, Mayo Clinic and other centers performed quantitative analyses of therapeutic responses in men and women with glioblastoma, and applied a computational algorithm to male and female transcriptome data.
"Our study indicates that in men receiving standard glioblastoma treatment, survival correlates with the expression of cell cycle regulators — whereas in women, the correlation is with the expression of integrin signaling pathway components," Dr. Swanson says. "Women with glioblastoma tend to survive longer. We think improved outcomes for all patients might be achieved by tailoring treatment to sex differences in molecular mechanisms."
Pinpointing seizure focus
Mayo Clinic has developed quantifiable imaging to identify seizure focus, paving the way for treatment for people with medically intractable epilepsy that is multifocal or poorly localized. "We are able to provide options for people with the most challenging cases of epilepsy," says Erik H. Middlebrooks, M.D., a neuroradiologist at Mayo Clinic in Jacksonville, Florida.
One of those options, deep brain stimulation (DBS), was recently approved by the Food and Drug Administration for epilepsy treatment. The target of DBS — the anterior nucleus of the thalamus (ANT) — is difficult to visualize in standard MRI sequences. Mayo Clinic has extensive experience with DBS and has developed imaging methods to better guide the procedure for optimal outcomes.
"When examining the existing clinical trials of DBS in epilepsy, off-target DBS placement is a real concern. The neuroimaging technology that we have developed allows us to be more accurate in electrode placement and to improve the programming of the devices to prevent seizures," Dr. Middlebrooks says.
وضع القطب الكهربائي في الموقع المحدد وبرمجته بدقة
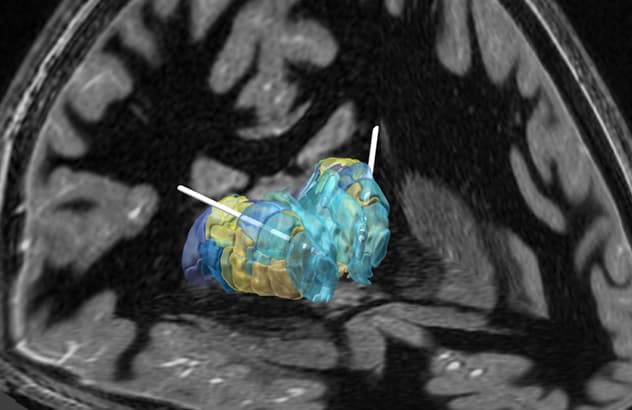
وضع القطب الكهربائي في الموقع المحدد وبرمجته بدقة
تسُهل عملية وضع القطب الكهربائي في الموقع المحدد وبرمجته بدقة عن طريق الجمع بين التصوير بالرنين المغناطيسي لالتقاط المادة الرمادية سريعًا، واستعادة عكس الإشارة T1 للاستهداف الجراحي مع تجزئة المهاد بناءً على اتصال الدماغ ووضع القطب آليًا.
In the August 2018 issue of Neurosurgical Focus, Mayo Clinic researchers described a novel use of the fast gray matter acquisition T1 inversion recovery MRI sequence to delineate the mammillothalamic tract for direct targeting of the ANT.
In a pilot study in the same Neurosurgical Focus issue, the researchers used functional MRI to demonstrate that successful ANT DBS in people with epilepsy correlated with increased connectivity in the default mode network and potential inhibition of the hippocampus — suggesting a potential mechanism of ANT DBS.
"We are very excited about the current DBS research taking place at Mayo Clinic. The field of brain 'connectomics' continues to revolutionize our understanding of the brain, and these developments are providing us important clues about which patients may benefit from DBS or other forms of neuromodulation," Dr. Middlebrooks says.
Another imaging innovation developed at Mayo Clinic is ictal single-photon emission computerized tomography (SPECT), which compares brain imaging taken during a seizure and at baseline. The baseline activity is subtracted from seizures to create images that can reveal seizure origin locations. Testing is performed in the epilepsy monitoring unit.
"Mayo remains fairly unique in offering ictal SPECT," says Benjamin (Ben) H. Brinkmann, Ph.D., a biomedical engineer in the Epilepsy and Neurophysiology Laboratory at Mayo Clinic's campus in Minnesota.
Morphometric MRI analysis (MAP), also developed at Mayo Clinic, can detect the subtle signs of focal cortical dysplasia. As described in the February 2018 issue of Epilepsy Research, MAP uses voxel-by-voxel comparisons between patient MRIs and a normative sample to detect abnormal thickening in cortical brain matter and abnormal blurring of the gray matter-white matter border. "Those signs of focal cortical dysplasia are very difficult to detect otherwise," Dr. Brinkmann says.
Stereo-electroencephalography (EEG), in which multiple electrodes are surgically implanted in the brain, can determine the source of epileptic activity by identifying the electrodes that are activated during a seizure. "Stereo-EEG imaging allows us to sample the deep brain areas — the cingulum, the insula — that we just couldn't record from before," Dr. Brinkmann says.
Mayo Clinic's imaging expertise rests on the enterprise's multidisciplinary approach, in which neurologists, neurosurgeons, neuroradiologists and neuropsychologists collaborate to improve patient care. "As neuroradiologists, we are an integral part of the multidisciplinary team," Dr. Middlebrooks says. "Imaging has become a pivotal diagnostic tool in the neurosciences and continues to be one of the key predictors of seizure freedom."
For more information
Korfiatis P, et al. Residual deep convolutional neural network predicts MGMT methylation status. Journal of Digital Imaging. 2017;30:622.
Yang W, et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Science Translational Medicine. 2019;11:eaao5253.
Grewal SS, et al. Fast gray matter acquisition T1 inversion recovery MRI to delineate the mammillothalamic tract for preoperative direct targeting of the anterior nucleus of the thalamus for deep brain stimulation in epilepsy. Neurosurgical Focus. 2018;45:E6.
Middlebrooks EH, et al. Differences in functional connectivity profiles as a predictor of response to anterior thalamic nucleus deep brain stimulation for epilepsy: A hypothesis for the mechanism of action and a potential biomarker for outcomes. Neurosurgical Focus. 2018;45:E7.
Wong-Kisiel LC, et al. Morphometric analysis on T1-weighted MRI complements visual MRI review in focal cortical dysplasia. Epilepsy Research. 2018;140:184.