Nov. 20, 2015
Statins are among the most widely prescribed drugs in the world, having been shown to markedly reduce adverse atherosclerotic cardiovascular (ASCVD) events in the primary and secondary prevention settings. Muscle and liver adverse effects, increased risk of diabetes, and the potential for drug interactions are limitations of this class of drugs.
Furthermore, patients on statins may be at substantial residual risk, highlighting the need for an alternative class of potent lipid-lowering agents. A cross-sectional analysis of a National Health and Nutrition Examination Survey (NHANES) dataset revealed low-density lipoprotein cholesterol (LDL-C) levels higher than 70 mg/dL in about 75 percent of patients at high ASCVD risk. The addition of ezetimibe and niacin may provide a moderate additional decrease in LDL-C; however, this may not be sufficient in patients with very high baseline LDL-C levels.
Hipercolesterolemia y anticuerpos monoclonales contra PCSK9
Hipercolesterolemia y anticuerpos monoclonales contra PCSK9
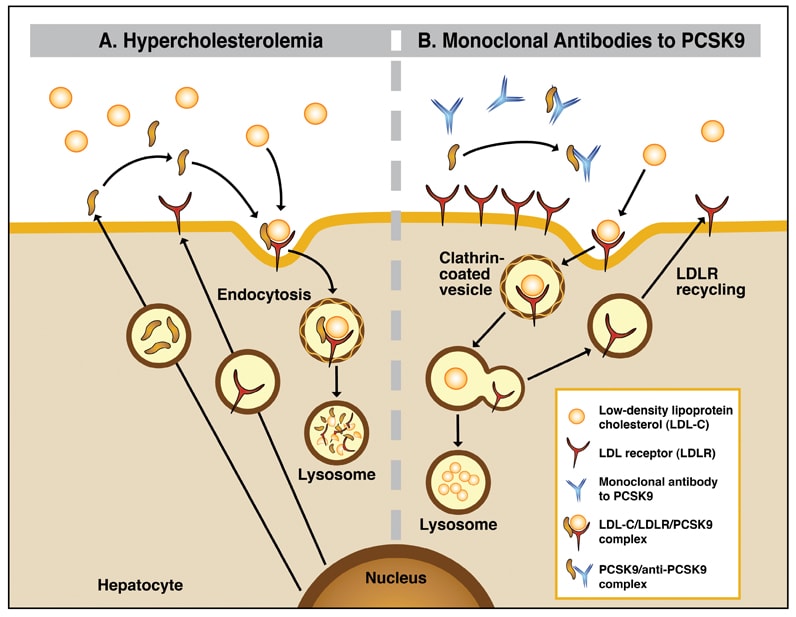
La serina proteasa de la proproteína convertasa subtilisina/kexina tipo 9 (PCSK9) desempeña una función importante en el metabolismo del colesterol al regular la degradación del receptor de lipoproteínas de baja densidad. La fijación de la PCSK9 circulante por los anticuerpos monoclonales parenterales tiene como resultado la recirculación aumentada del receptor de LDL hacia la superficie de hepatocitos y el aclaramiento acelerado del C-LDL circulante.
Proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) plays an important role in cholesterol metabolism by regulating LDL receptor degradation.
Shortly after its discovery in 2001, the gene encoding PCSK9 was implicated in familial hypercholesterolemia (FH). Gain and loss-of-function variations in PCSK9 resulted in high and low levels of LDL-C, respectively. Genetically determined decreases in LDL-C levels — 28 percent in African Americans and 15 percent in white subjects — were associated with a substantial reduction in the coronary heart disease risk as compared with LDL-C levels in noncarriers — 88 percent and 47 percent, respectively. These findings triggered a race to develop PCSK9 inhibitors to reduce LDL-C for prevention of ASCVD-related adverse events.
Binding of circulating PCSK9 by parenteral monoclonal antibodies results in augmented recirculation of the LDL receptor to the hepatocyte surface and accelerated clearance of circulating LDL-C.
First tested in animals in 2009, anti-PCSK9 antibodies have shown a significant lipid-lowering effect comparable to that of lipoprotein apheresis, that is, a 55 to 75 percent decrease from baseline regardless of whether patients are on statins or ezetimibe. More than 70 percent of high-risk patients are able to achieve an LDL-C level less than 70 mg/dL.
In placebo-controlled trials of patients on a maximally tolerated statin dose, LDL-C reduction was twice that achieved with ezetimibe, and the lipid-lowering effect was not affected by age, sex, intensity of statin regimen, ASCVD risk or presence of diabetes.
Data demonstrating the safety and efficacy of PCSK9 inhibitors led to the recent Food and Drug Administration approval of two human monoclonal antibodies:
- Alirocumab (Praluent), 75-150 mg every two weeks
- Evolocumab (Repatha), 140 mg every two weeks or 420 mg monthly
The drugs are approved for the following indications:
- Patients with heterozygous FH
- Individuals with ASCVD, who require additional LDL-C lowering
Evolocumab, 420 mg once monthly, is additionally approved for homozygous FH based on results of the TESLA trial, in which LDL-C levels were reduced by 18 to 44 percent with monthly injections during a three-month period in 50 homozygous FH patients showing at least 2 percent of functioning LDL receptors.
In the longer term and extension trials, the lipid-lowering effects were enduring and consistent with those attained at three and six months. Additionally, a significant reduction in apolipoprotein B, non-high-density lipoprotein cholesterol (non-HDL-C) and lipoprotein(a) (about 25 to 30 percent decrease) as well as modest favorable effect on HDL-C and triglyceride levels has been observed with anti-PCSK9 drugs. The mechanisms by which PCSK9 inhibitors decrease levels of lipoprotein(a) are unknown at present.
Two simultaneously published studies — the ODYSSEY long-term study carried out in 2,341 patients at high risk of ASCVD events on maximally tolerated statin dose and the OSLER study in 4,465 patients, including those at high risk of ASCVD— showed comparable decreases of 60 percent in LDL-C level from the mean of 120 mg/dL for both evolocumab and alirocumab. This effect was associated with a halving of the rate of composite ASCVD end points in a post hoc analysis.
Placebo- as well as ezetimibe-controlled trials in patients with statin intolerance showed no excess in adverse events such as insulin resistance, glucose intolerance or myopathy. Common adverse effects (seen in ≥ 5 percent of treated patients) included upper respiratory tract infections, nasopharyngitis, influenza and injection site reactions. For evolocumab, back pain was additionally described.
Reassuringly, anti-drug antibody development has not yet been reported. The frequencies of serious treatment-related adverse events are not different from placebo, although potential for neurocognitive effects is unclear.
"PCSK9 inhibitors fill an obvious therapeutic niche in selective high-risk patients, such as FH or statin-intolerant patients, who are not able to achieve the desired LDL-C level with conventional treatments," according to Iftikhar J. Kullo, M.D., a cardiologist at Mayo Clinic in Rochester, Minnesota. However, objective criteria to determine who should get these drugs are yet to be established.
Another important consideration is cost, which is comparable to lipoprotein apheresis and antisense oligonucleotide technologies but far greater than statin therapy. Results of large outcome studies of PCSK9 inhibition are eagerly awaited by the medical community and patients.