Diagnosis
Bone marrow exam
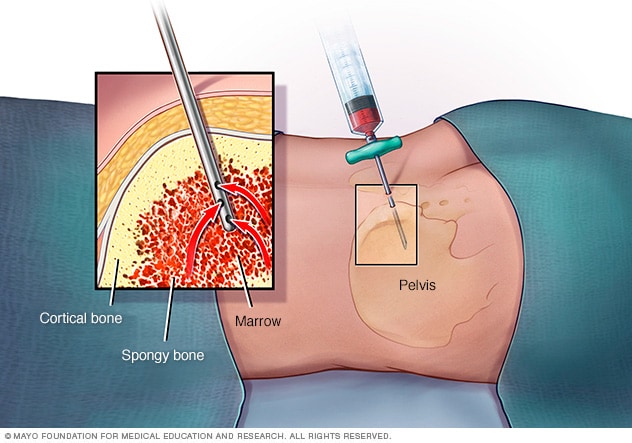
Bone marrow exam
In a bone marrow aspiration, a health care provider uses a thin needle to remove a small amount of liquid bone marrow, usually from a spot in the back of your hipbone (pelvis). A bone marrow biopsy is often done at the same time. This second procedure removes a small piece of bone tissue and the enclosed marrow.
Doctors may find chronic leukemia in a routine blood test, before symptoms begin. If this happens, or if you have signs or symptoms that suggest leukemia, you may undergo the following diagnostic exams:
- Physical exam. Your doctor will look for physical signs of leukemia, such as pale skin from anemia, swelling of your lymph nodes, and enlargement of your liver and spleen.
- Blood tests. By looking at a sample of your blood, your doctor can determine if you have abnormal levels of red or white blood cells or platelets — which may suggest leukemia. A blood test may also show the presence of leukemia cells, though not all types of leukemia cause the leukemia cells to circulate in the blood. Sometimes the leukemia cells stay in the bone marrow.
- Bone marrow test. Your doctor may recommend a procedure to remove a sample of bone marrow from your hipbone. The bone marrow is removed using a long, thin needle. The sample is sent to a laboratory to look for leukemia cells. Specialized tests of your leukemia cells may reveal certain characteristics that are used to determine your treatment options.
Treatment
Treatment for your leukemia depends on many factors. Your doctor determines your leukemia treatment options based on your age and overall health, the type of leukemia you have, and whether it has spread to other parts of your body, including the central nervous system.
Common treatments used to fight leukemia include:
-
Chemotherapy. Chemotherapy is the major form of treatment for leukemia. This drug treatment uses chemicals to kill leukemia cells.
Depending on the type of leukemia you have, you may receive a single drug or a combination of drugs. These drugs may come in a pill form, or they may be injected directly into a vein.
- Targeted therapy. Targeted drug treatments focus on specific abnormalities present within cancer cells. By blocking these abnormalities, targeted drug treatments can cause cancer cells to die. Your leukemia cells will be tested to see if targeted therapy may be helpful for you.
-
Radiation therapy. Radiation therapy uses X-rays or other high-energy beams to damage leukemia cells and stop their growth. During radiation therapy, you lie on a table while a large machine moves around you, directing the radiation to precise points on your body.
You may receive radiation in one specific area of your body where there is a collection of leukemia cells, or you may receive radiation over your whole body. Radiation therapy may be used to prepare for a bone marrow transplant.
-
Bone marrow transplant. A bone marrow transplant, also called a stem cell transplant, helps reestablish healthy stem cells by replacing unhealthy bone marrow with leukemia-free stem cells that will regenerate healthy bone marrow.
Prior to a bone marrow transplant, you receive very high doses of chemotherapy or radiation therapy to destroy your leukemia-producing bone marrow. Then you receive an infusion of blood-forming stem cells that help rebuild your bone marrow.
You may receive stem cells from a donor or you may be able to use your own stem cells.
- Immunotherapy. Immunotherapy uses your immune system to fight cancer. Your body's disease-fighting immune system may not attack your cancer because the cancer cells produce proteins that help them hide from the immune system cells. Immunotherapy works by interfering with that process.
- Engineering immune cells to fight leukemia. A specialized treatment called chimeric antigen receptor (CAR)-T cell therapy takes your body's germ-fighting T cells, engineers them to fight cancer and infuses them back into your body. CAR-T cell therapy might be an option for certain types of leukemia.
- Clinical trials. Clinical trials are experiments to test new cancer treatments and new ways of using existing treatments. While clinical trials give you or your child a chance to try the latest cancer treatment, treatment benefits and risks may be uncertain. Discuss the benefits and risks of clinical trials with your doctor.
More Information
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Coping and support
A diagnosis of leukemia may be devastating — especially for the family of a newly diagnosed child. With time you'll find ways to cope with the distress and uncertainty of cancer. Until then, you may find it helps to:
-
Learn enough about leukemia to make decisions about your care. Ask your doctor about your leukemia, including your treatment options and, if you like, your prognosis. As you learn more about leukemia, you may become more confident in making treatment decisions.
The term "leukemia" can be confusing because it refers to a group of cancers that aren't all that similar except for the fact that they affect the bone marrow and blood. You can waste a lot of time researching information that doesn't apply to your kind of leukemia. To avoid that, ask your doctor to write down as much information about your specific disease as possible. Then narrow your search for information accordingly.
- Keep friends and family close. Keeping your close relationships strong will help you deal with your leukemia. Friends and family can provide the practical support you'll need, such as helping take care of your house if you're in the hospital. And they can serve as emotional support when you feel overwhelmed by cancer.
-
Find someone to talk with. Find a good listener who is willing to listen to you talk about your hopes and fears. This may be a friend or family member. The concern and understanding of a counselor, medical social worker, clergy member or cancer support group also may be helpful.
Ask your doctor about support groups in your area. Or check your phone book, library or a cancer organization, such as the National Cancer Institute, the American Cancer Society or the Leukemia & Lymphoma Society.
- Take care of yourself. It's easy to get caught up in the tests, treatments and procedures of therapy. But it's important to take care of yourself, not just the cancer. Try to make time for yoga, cooking or other favorite diversions.
Preparing for your appointment
Start by seeing your family doctor if you have signs or symptoms that worry you. If your doctor suspects you have leukemia, you may be referred to a doctor who specializes in diseases of the blood and bone marrow (hematologist).
Because appointments can be brief, and because there's often a lot of information to discuss, it's a good idea to be prepared. Here's some information to help you get ready, and know what to expect from your doctor.
What you can do
- Be aware of any pre-appointment restrictions. At the time you make the appointment, be sure to ask if there's anything you need to do in advance, such as restrict your diet.
- Write down any symptoms you're experiencing, including any that may seem unrelated to the reason for which you scheduled the appointment.
- Write down key personal information, including any major stresses or recent life changes.
- Make a list of all medications, vitamins or supplements that you're taking.
- Consider taking a family member or friend along. Sometimes it can be difficult to remember all the information provided during an appointment. Someone who accompanies you may remember something that you missed or forgot.
- Write down questions to ask your doctor.
Your time with your doctor is limited, so preparing a list of questions can help you make the most of your time together. List your questions from most important to least important in case time runs out. For leukemia, some basic questions to ask your doctor include:
- Do I have leukemia?
- What type of leukemia do I have?
- Do I need more tests?
- Does my leukemia need immediate treatment?
- What are the treatment options for my leukemia?
- Can any treatments cure my leukemia?
- What are the potential side effects of each treatment option?
- Is there one treatment you feel is best for me?
- How will treatment affect my daily life? Can I continue working or going to school?
- I have these other health conditions. How can I best manage them together?
- Should I see a specialist? What will that cost, and will my insurance cover it?
- Are there brochures or other printed material that I can take with me? What websites do you recommend?
In addition to the questions that you've prepared to ask your doctor, don't hesitate to ask other questions during your appointment.
What to expect from your doctor
Your doctor is likely to ask you a number of questions. Being ready to answer them may allow more time later to cover other points you want to address. Your doctor may ask:
- When did you first begin experiencing symptoms?
- Have your symptoms been continuous or occasional?
- How severe are your symptoms?
- What, if anything, seems to improve your symptoms?
- What, if anything, appears to worsen your symptoms?
- Have you ever had abnormal blood test results? If so, when?
Sept. 21, 2022